Hypotensive drug urapidil hydrochloride composition freeze-dried powder injection
A technology of freeze-dried powder injection and urapidil, which is applied in the field of medicine, can solve problems such as poor stability, toxicity to patients, and difficult storage, and achieve good stability, improved fluidity, and low content of insoluble particles.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
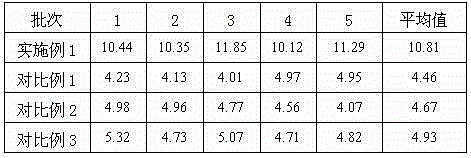
Embodiment 1
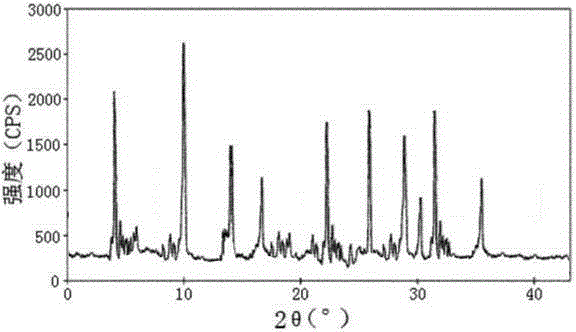
[0031] Embodiment 1: the preparation of urapidil crystal
[0032] (1) Grind the crude product of urapidil, pass it through an 80-mesh sieve, then add it into methanol whose volume is 6 times the weight of uradil, and stir at 130 rpm for 10 minutes;
[0033] (2) Add acetone with a volume 4 times the weight of urapidil under stirring at 90 rpm, and raise the temperature to 35°C at the same time;
[0034] (3) After the solution is added, let it stand for 3 hours, and add dropwise a mixed solution of petroleum ether and carbon tetrachloride with a volume of 8 times the weight of uradil at 0°C under the condition of stirring at 150 rpm, petroleum ether, tetrachloride The volume ratio of carbon chloride is 3:1, and the uniform dropwise addition is completed within 2 hours;
[0035] (4) After the dropwise addition, the temperature was lowered to -5°C, and the stirring was continued at a stirring rate of 110 rpm for 2 h, and the crystals were precipitated after standing for 1 h, filt...
Embodiment 2
[0037] Embodiment 2: the preparation of urapidil freeze-dried powder injection
[0038] Prescription: 3 parts by weight of urapidil crystal form compound prepared in Example 1, 15 parts by weight of trehalose;
[0039] Preparation steps: take the urapidil compound of the present invention, stir and dissolve it with water for injection, add the prescribed amount of trehalose, adjust the pH value to 5.0-6.5, then stir until the pH remains constant, then add water for injection until the volume of the solution is uradil 100 times its weight, then coarsely filter with activated carbon, pass through 1.0μm, 0.45μm, 0.22μm microporous membranes in turn to sterilize and filter, filter into a sterile room, measure the pH and content to pass, fill, and press half the plug , put it into a freeze-drying box that has been cooled to -25°C, freeze-dry at low temperature, press the plug out of the box, and roll the cover.
[0040] Described freeze-drying is:
[0041] Pre-freezing: freeze-dr...
Embodiment 3
[0044] Embodiment 3: the preparation of urapidil freeze-dried powder injection
[0045] Prescription: 3 parts by weight of urapidil crystal form compound prepared in Example 1, 18 parts by weight of trehalose;
[0046]Preparation steps: take the urapidil compound of the present invention, stir and dissolve it with water for injection, add the prescribed amount of trehalose, adjust the pH value to 5.0-6.5, then stir until the pH remains constant, then add water for injection until the volume of the solution is uradil 100 times its weight, then coarsely filter with activated carbon, pass through 1.0μm, 0.45μm, 0.22μm microporous membranes in turn to sterilize and filter, filter into a sterile room, measure the pH and content to pass, fill, and press half the plug , put it into a freeze-drying box that has been cooled to -25°C, freeze-dry at low temperature, press the plug out of the box, and roll the cover.
[0047] Described freeze-drying is:
[0048] Pre-freezing: freeze-dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com