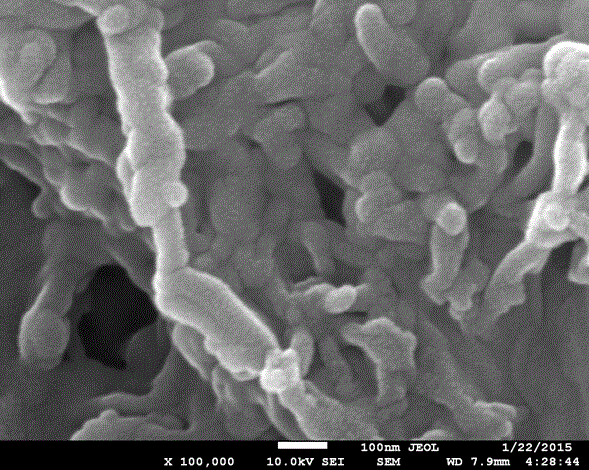
Preparation method of biomimetic bone material containing graphene, hydroxyapatite and fibroin
A technology of hydroxyapatite and biomimetic materials, applied in medical science, prostheses, etc., can solve problems such as lack of biological activity, poor hydrophilicity, inflammation, etc., achieve good cell affinity, good biocompatibility, and improve Effects of Compressive Strength and Toughness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A preparation method of a nano-bone biomimetic material containing graphene, hydroxyapatite and silk fibroin, comprising the following steps:
[0026] (1) Ultrasonic dispersion of graphene oxide into 0.5 mol / L sodium hydroxide ethanol (85%) solution with a bath ratio of 1:500, alkalized at 30°C for 60 min. Then add 0.725mol / L sodium chloroacetate solution, ultrasonically stir at 60°C for 50min, adjust the pH value of the solution to neutral with 0.1mol / L hydrochloric acid and dialyze in distilled water for three days to obtain carboxylated graphene aqueous solution;
[0027] (2) According to the calcium-phosphorus molar ratio of 1.67, 0.05mol / L Ca(OH) 2 with diluted H 3 PO 4 Use a burette to drop the solution into the solution prepared in step (1) at the same time and stir in ultrasonic for 4 hours, add ammonia water drop by drop to control the reaction pH to 8.0-9.0, and control the reaction temperature to 60°C to obtain graphene / hydroxyapatite Composite particle su...
Embodiment 2
[0033] A preparation method of a nano-bone biomimetic material containing graphene, hydroxyapatite and silk fibroin, comprising the following steps:
[0034] (1) Ultrasonic dispersion of graphene oxide into 1mol / L sodium hydroxide ethanol (85%) solution with a bath ratio of 1:500, alkalized at 50°C for 20min. Then add 1.25mol / L sodium chloroacetate solution, ultrasonically stir for 100min at 50°C, adjust the pH value of the solution to neutral with 0.3mol / L hydrochloric acid and dialyze in distilled water for three days to obtain carboxylated graphene aqueous solution;
[0035] (2) According to the calcium-phosphorus molar ratio of 1.67, 0.1mol / L Ca(OH) 2 with diluted H 3 PO 4 Use a burette to drop the solution into the solution prepared in step (1) at the same time and stir in ultrasonic for 2 hours, add ammonia water drop by drop to control the reaction pH to 8.0-10.0, and control the reaction temperature to 75°C to obtain graphene / hydroxyapatite Composite particle suspen...
Embodiment 3
[0040] A preparation method of a nano-bone biomimetic material containing graphene, hydroxyapatite and silk fibroin, comprising the following steps:
[0041] (1) Ultrasonically disperse graphene oxide into 0.08mol / L sodium hydroxide ethanol (85%) solution with a bath ratio of 1:500, and basify at 40°C for 30min. Then add 0.1mol / L sodium chloroacetate solution, ultrasonically stir at 55°C for 60min, adjust the pH value of the solution to neutral with 0.2mol / L hydrochloric acid and dialyze in distilled water for three days to obtain carboxylated graphene aqueous solution;
[0042] (2) According to the calcium-phosphorus molar ratio of 1.67, 0.03mol / L Ca(OH) 2 with diluted H 3 PO 4 Use a burette to drop the solution into the solution prepared in step (1) at the same time and stir in ultrasonic for 3 hours, add ammonia water drop by drop to control the reaction pH to 8.0-10.0, and control the reaction temperature to 80°C to obtain graphene / hydroxyapatite Composite particle susp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com