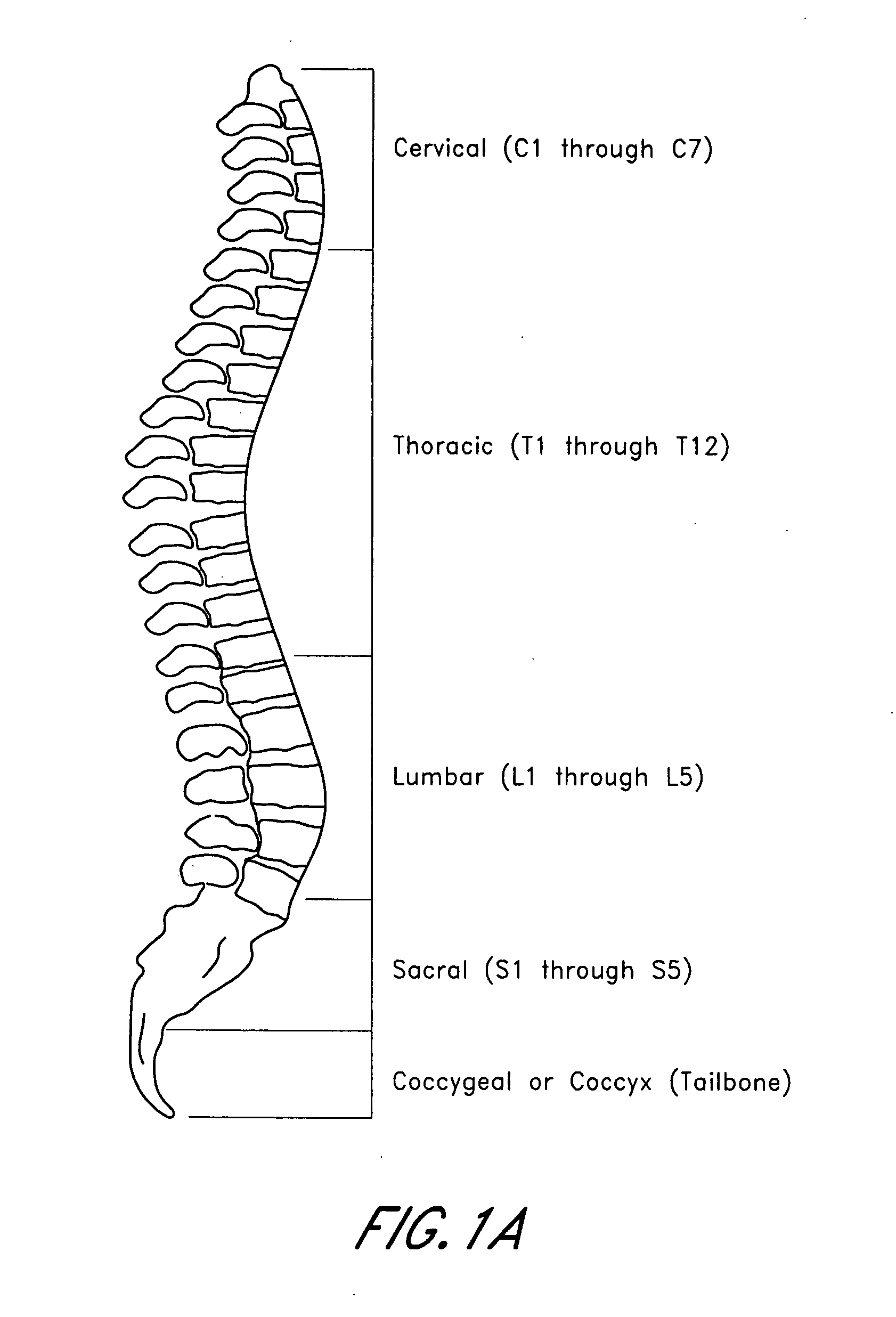
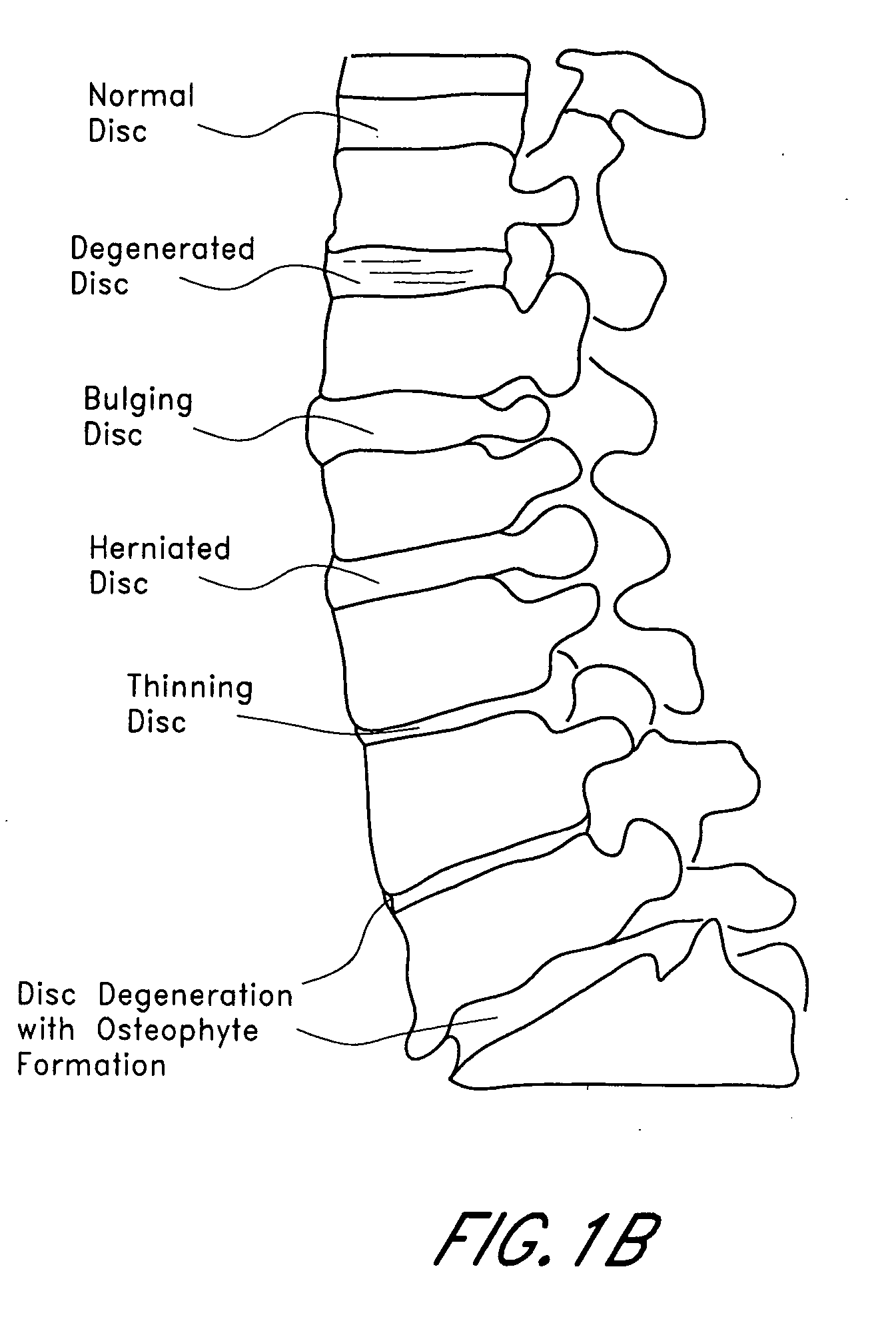
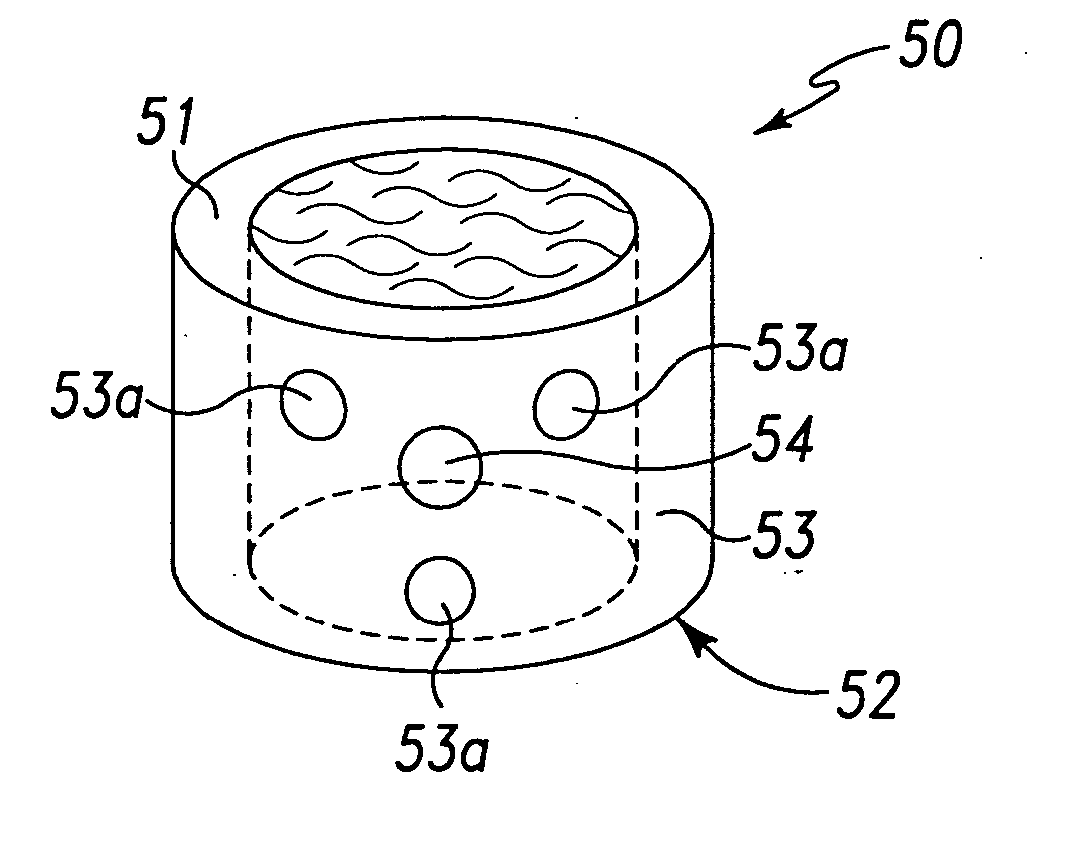
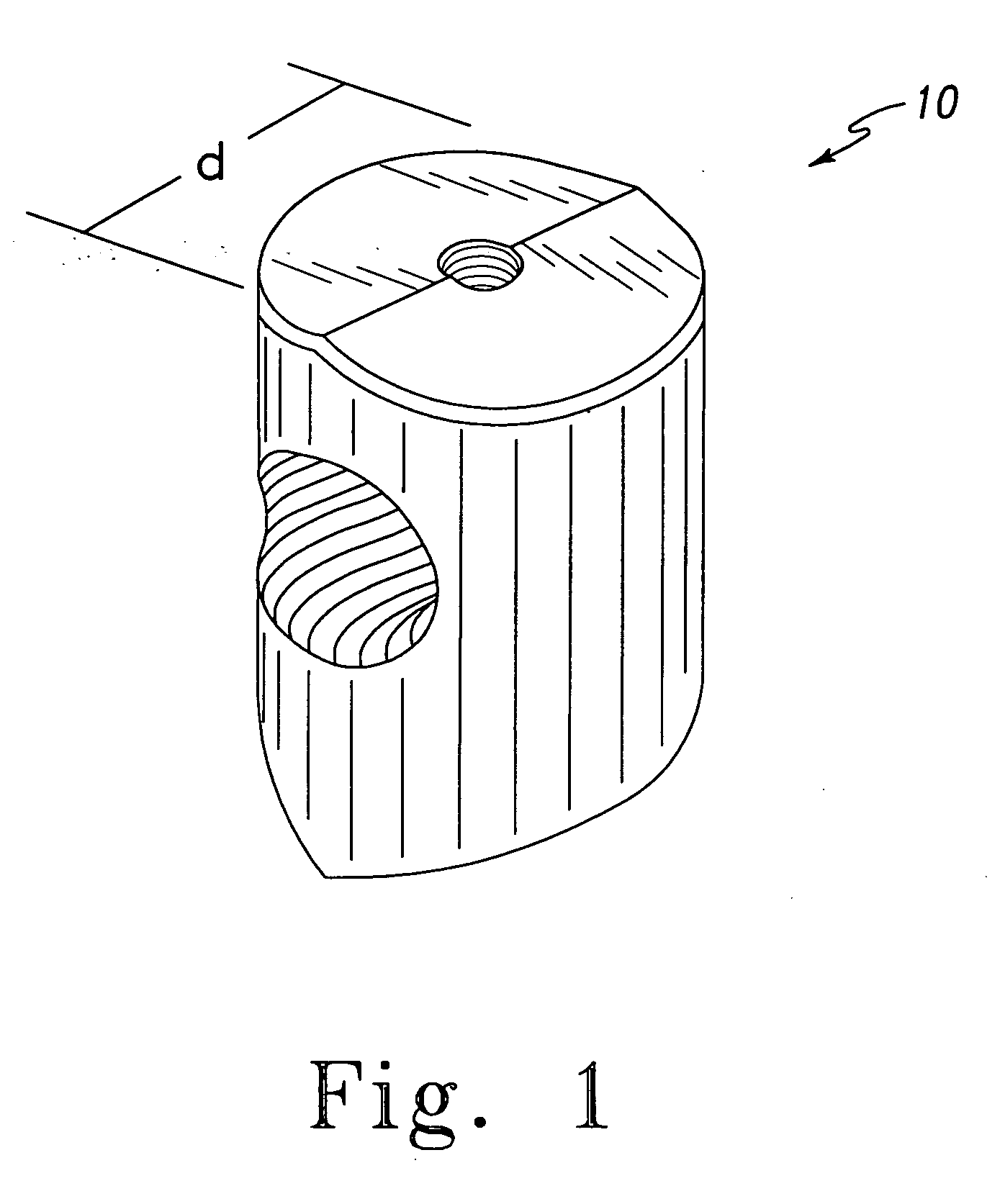
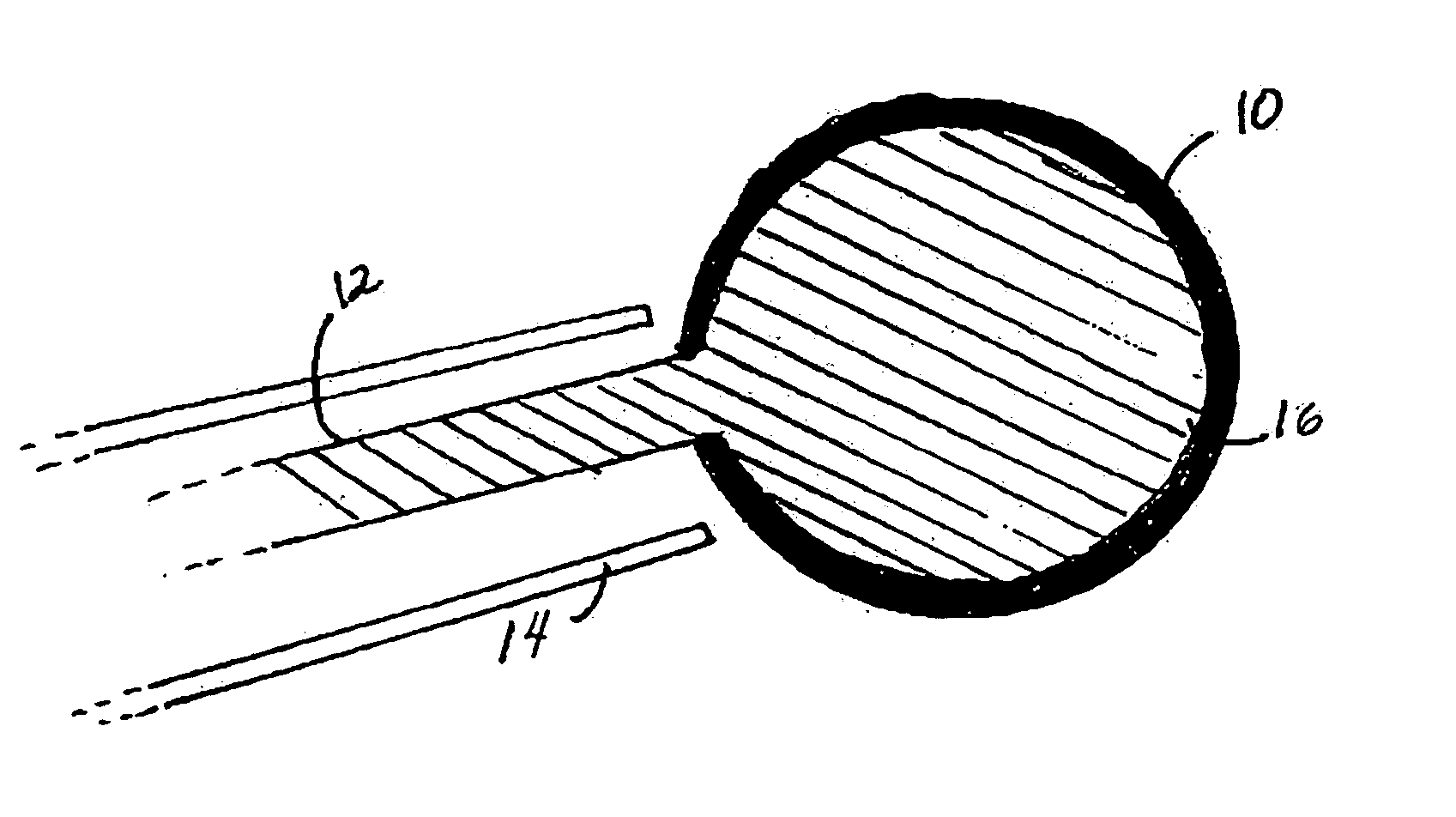
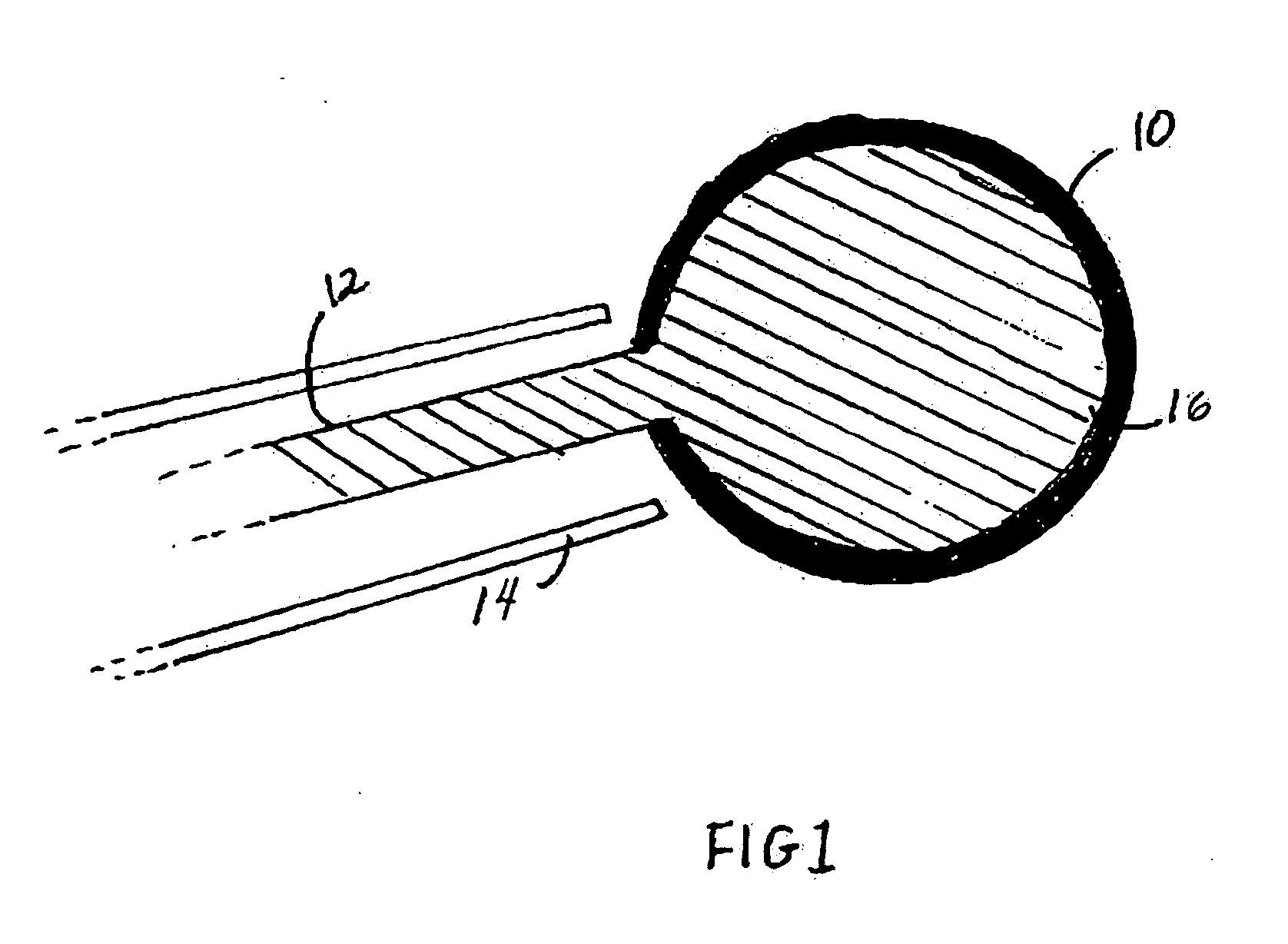
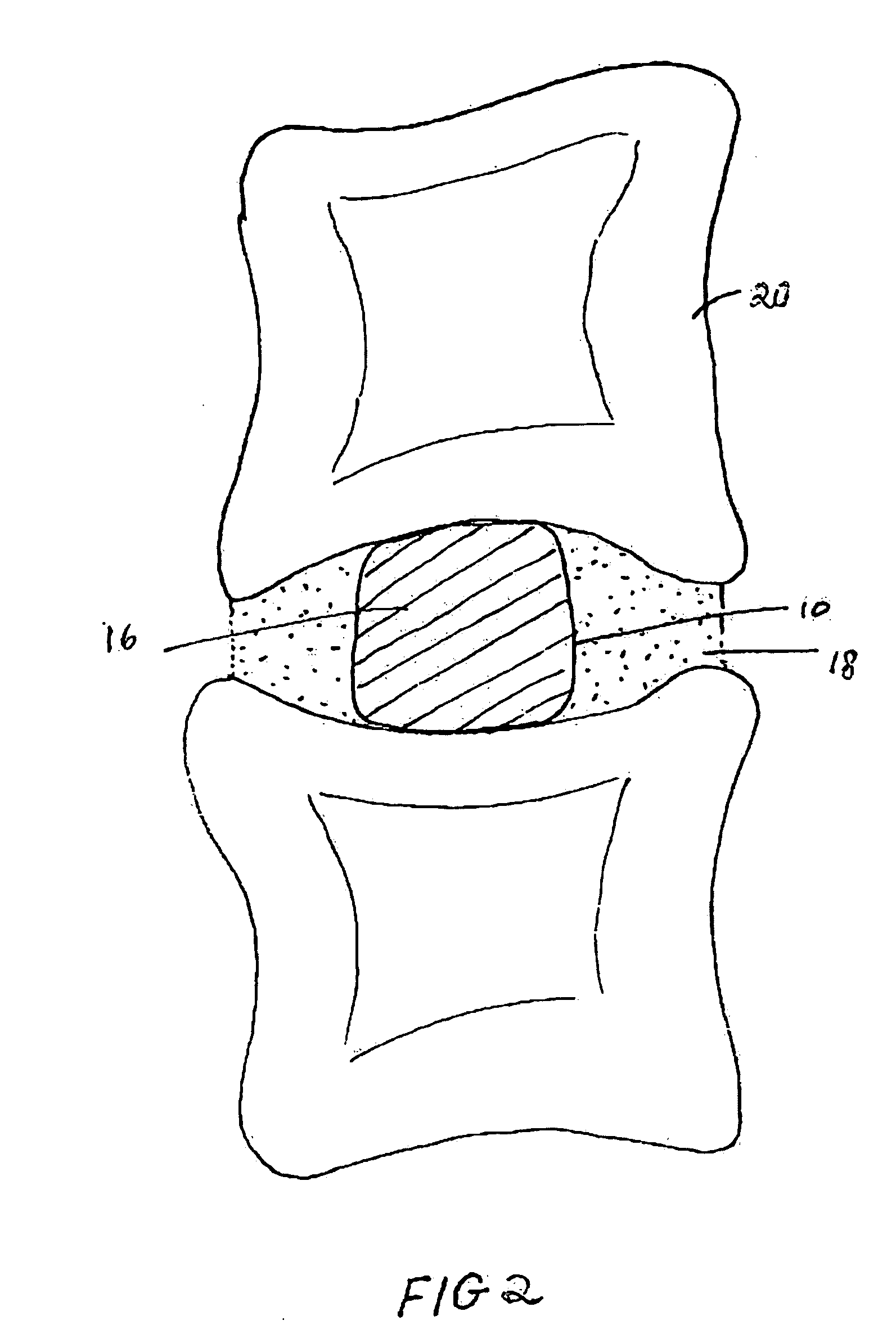
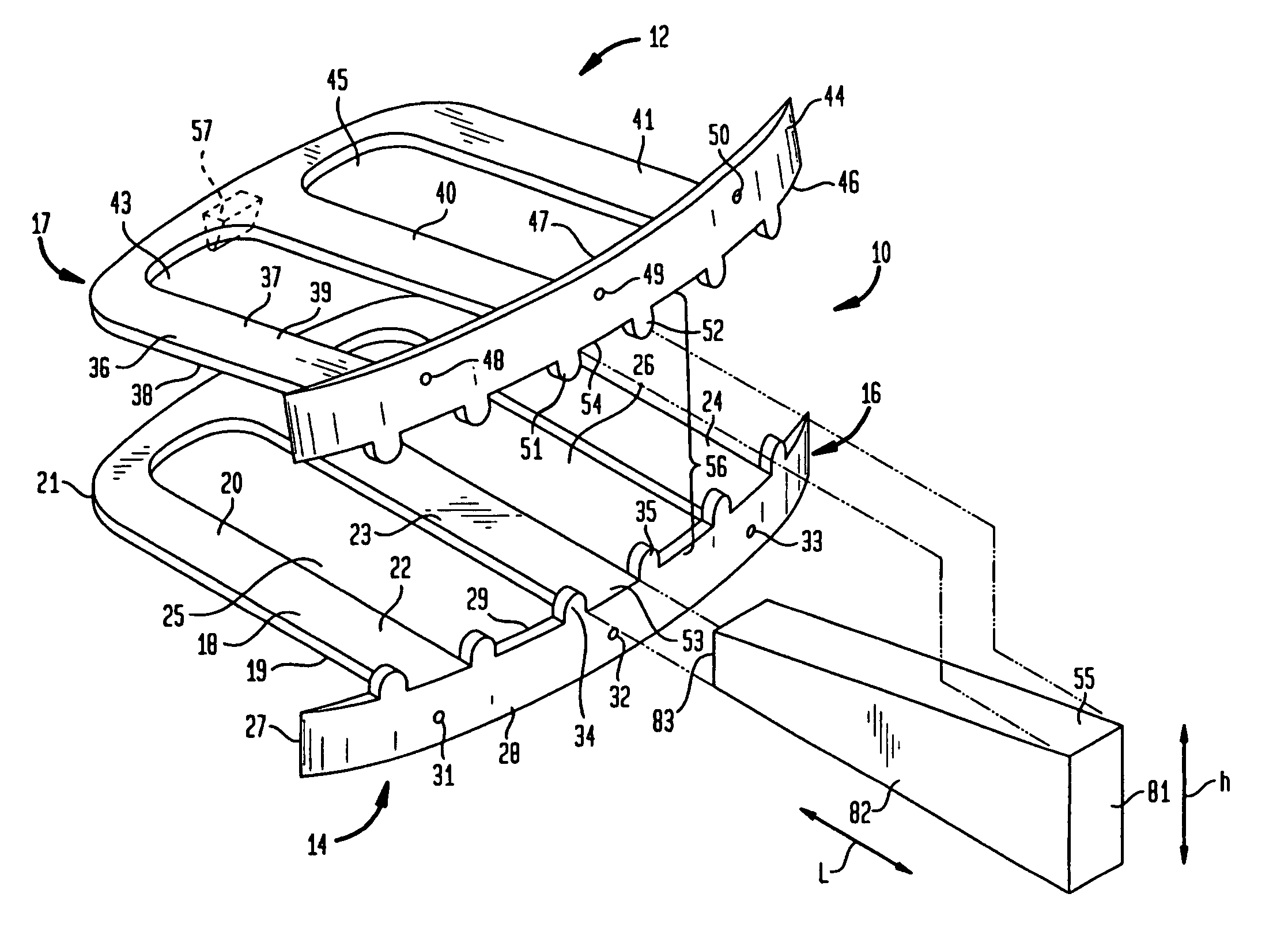
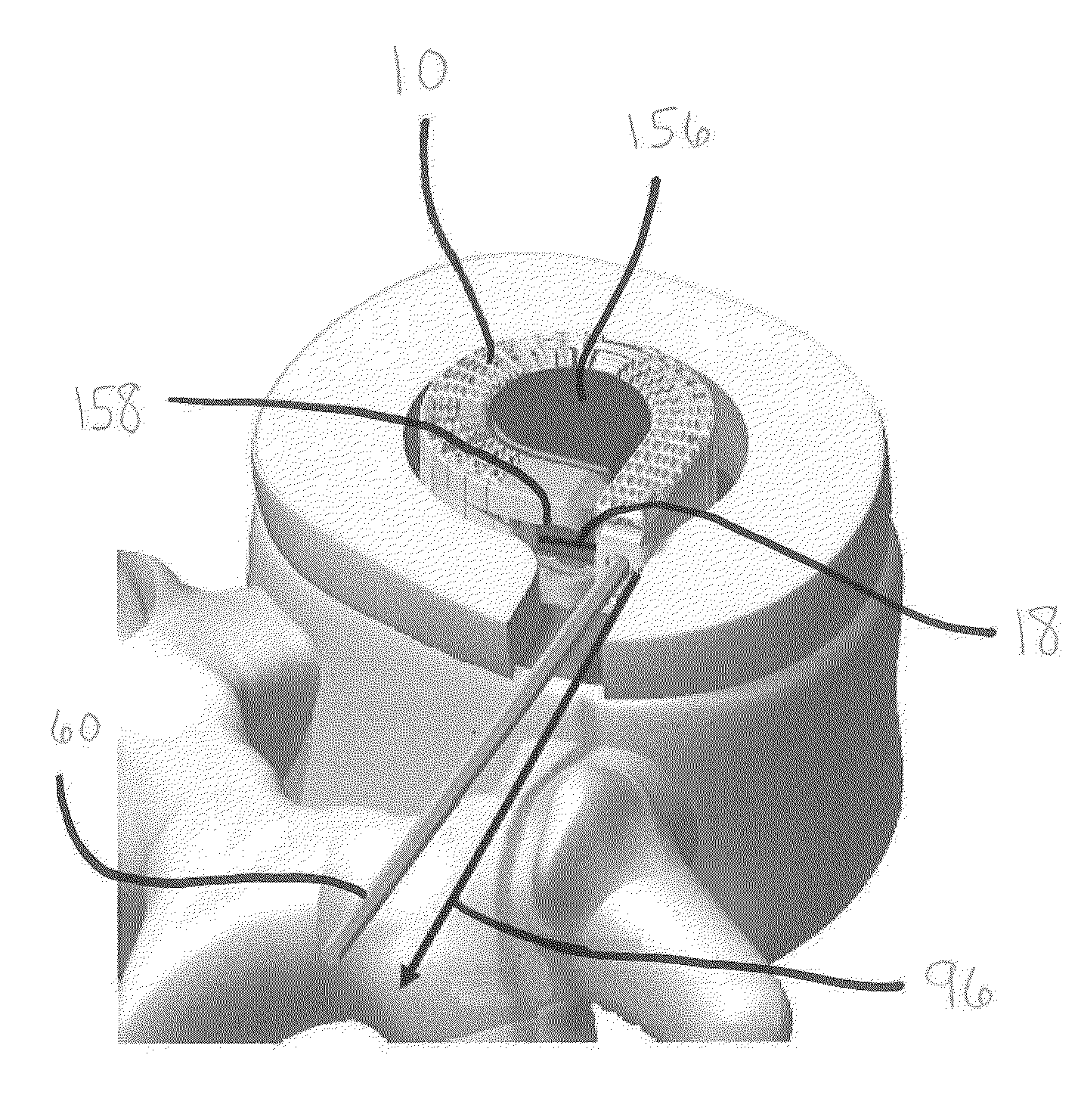
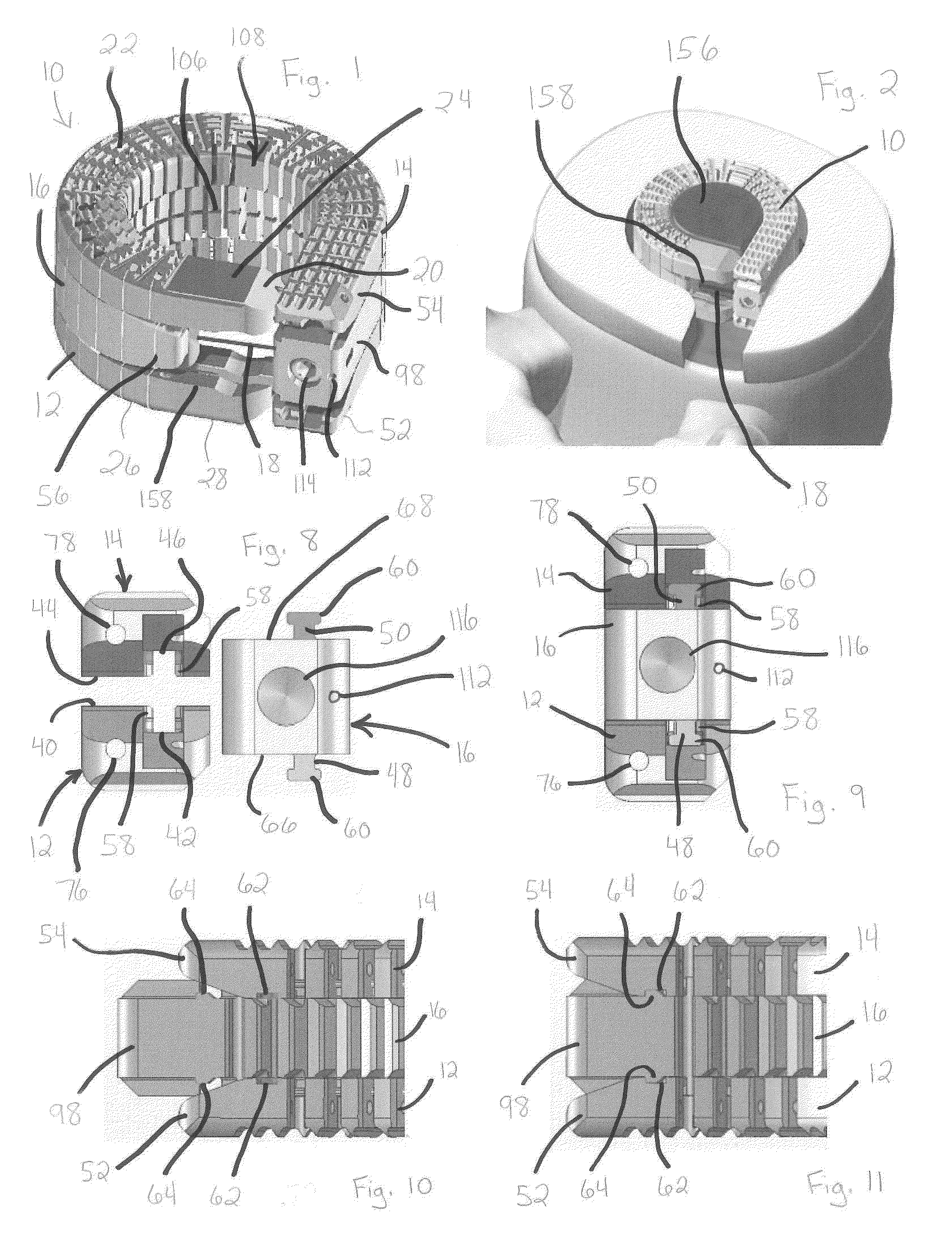
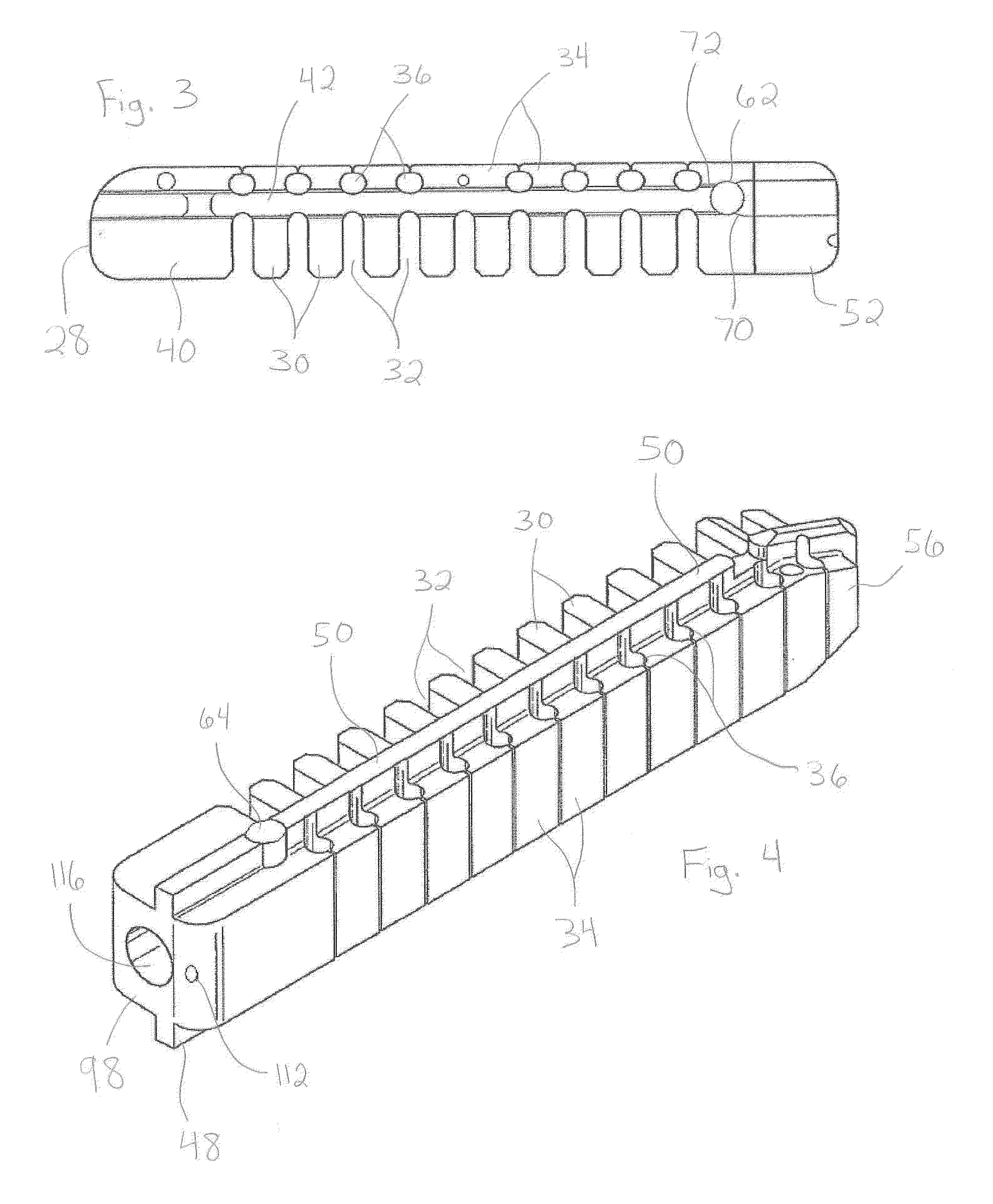
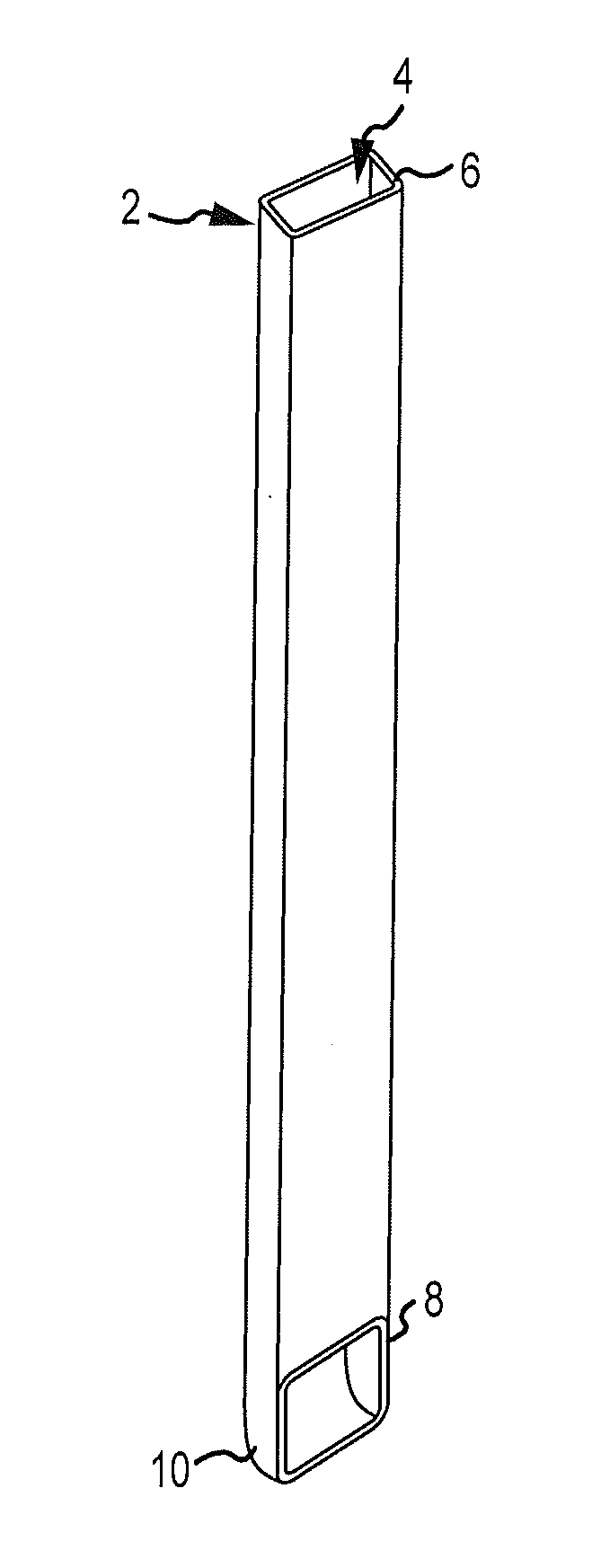
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
383 results about "Bone transplantation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
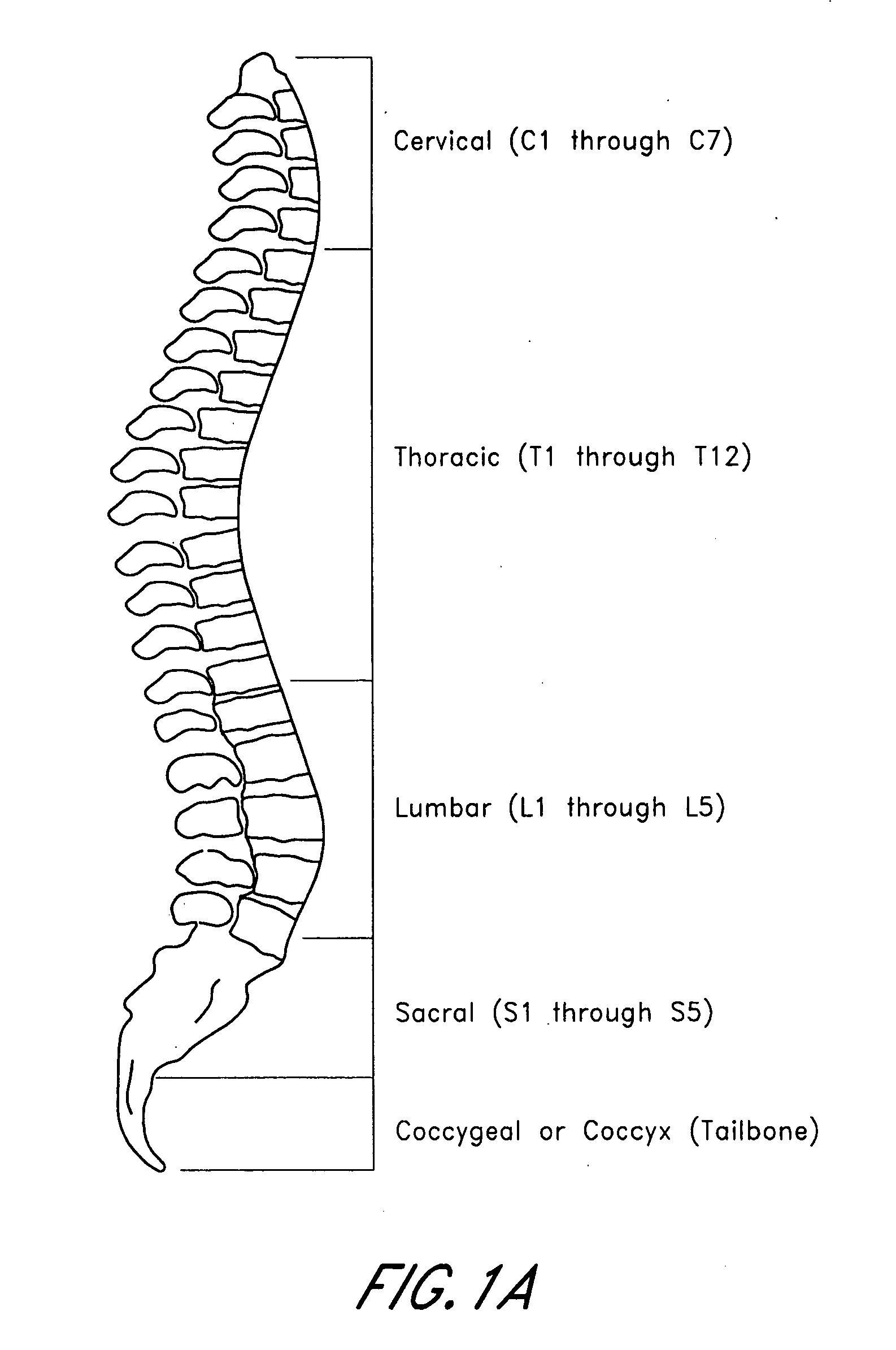
A bone marrow transplant is a blood and bone marrow stem cell transplant from a healthy donor to a recipient whose own bone marrow is affected by disease. Bone marrow transplant replaces a person’s abnormal stem cells with healthy ones from another person (a donor). Bone marrow is the soft, sponge-like material found inside bones.
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
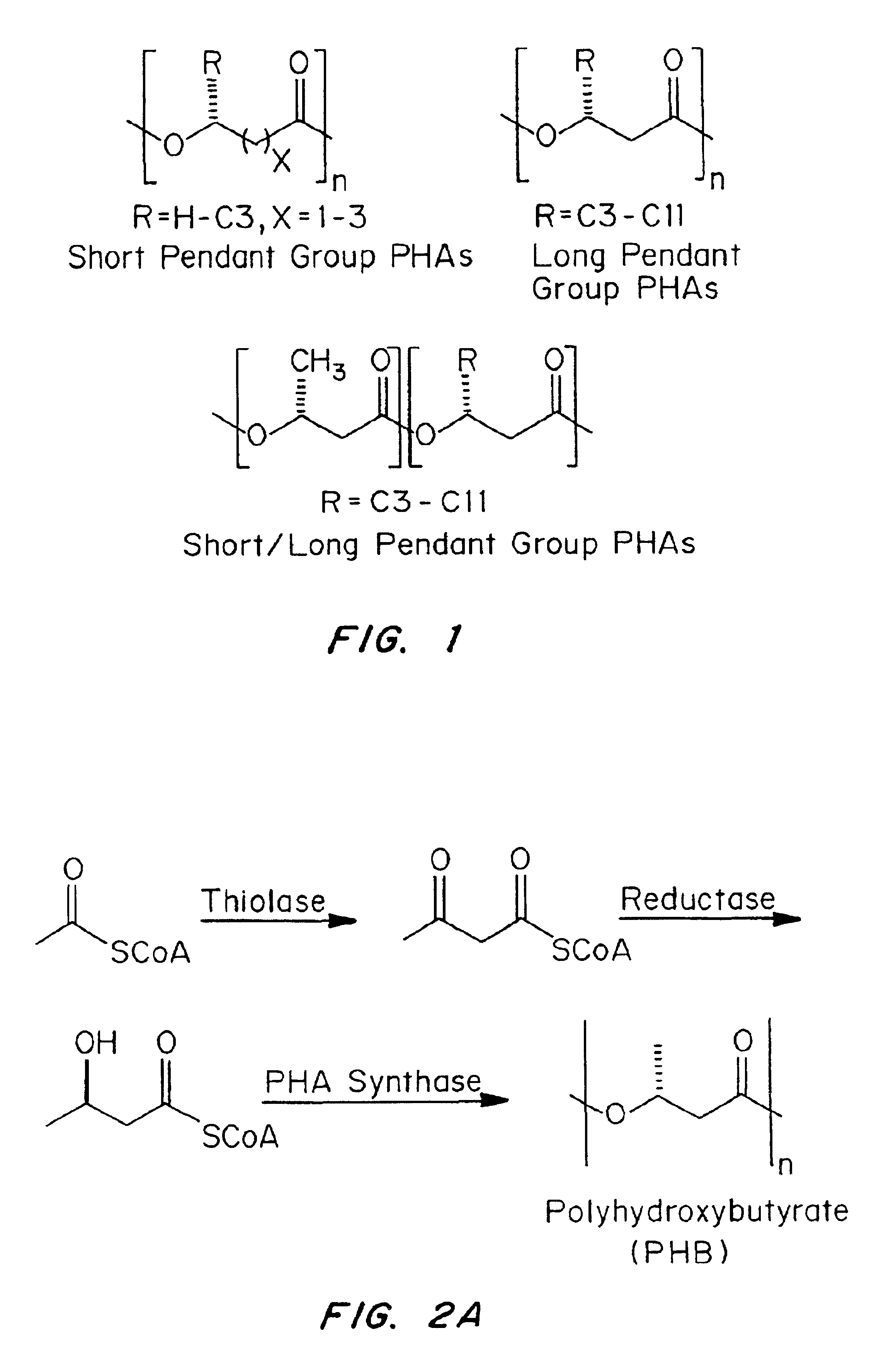
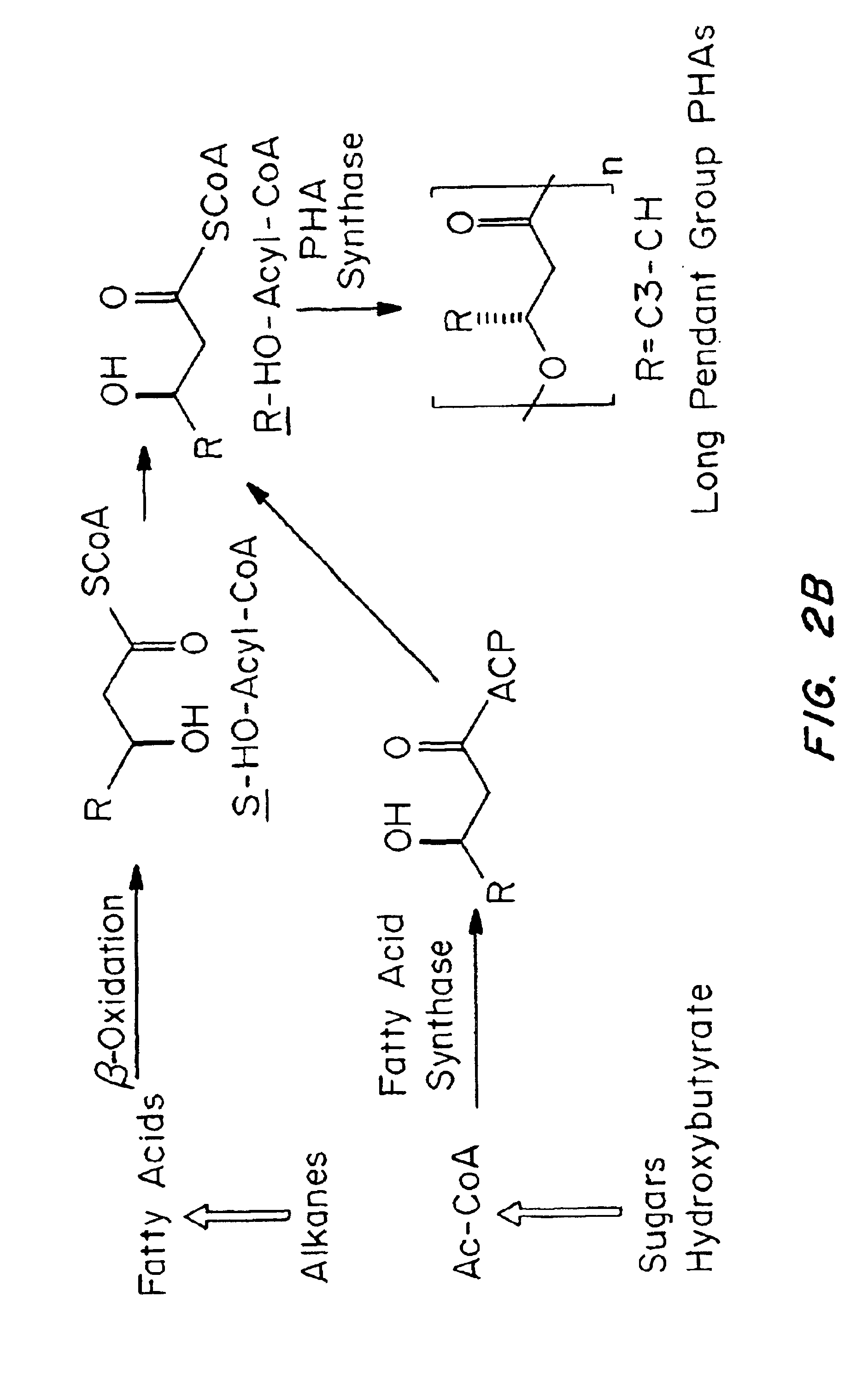
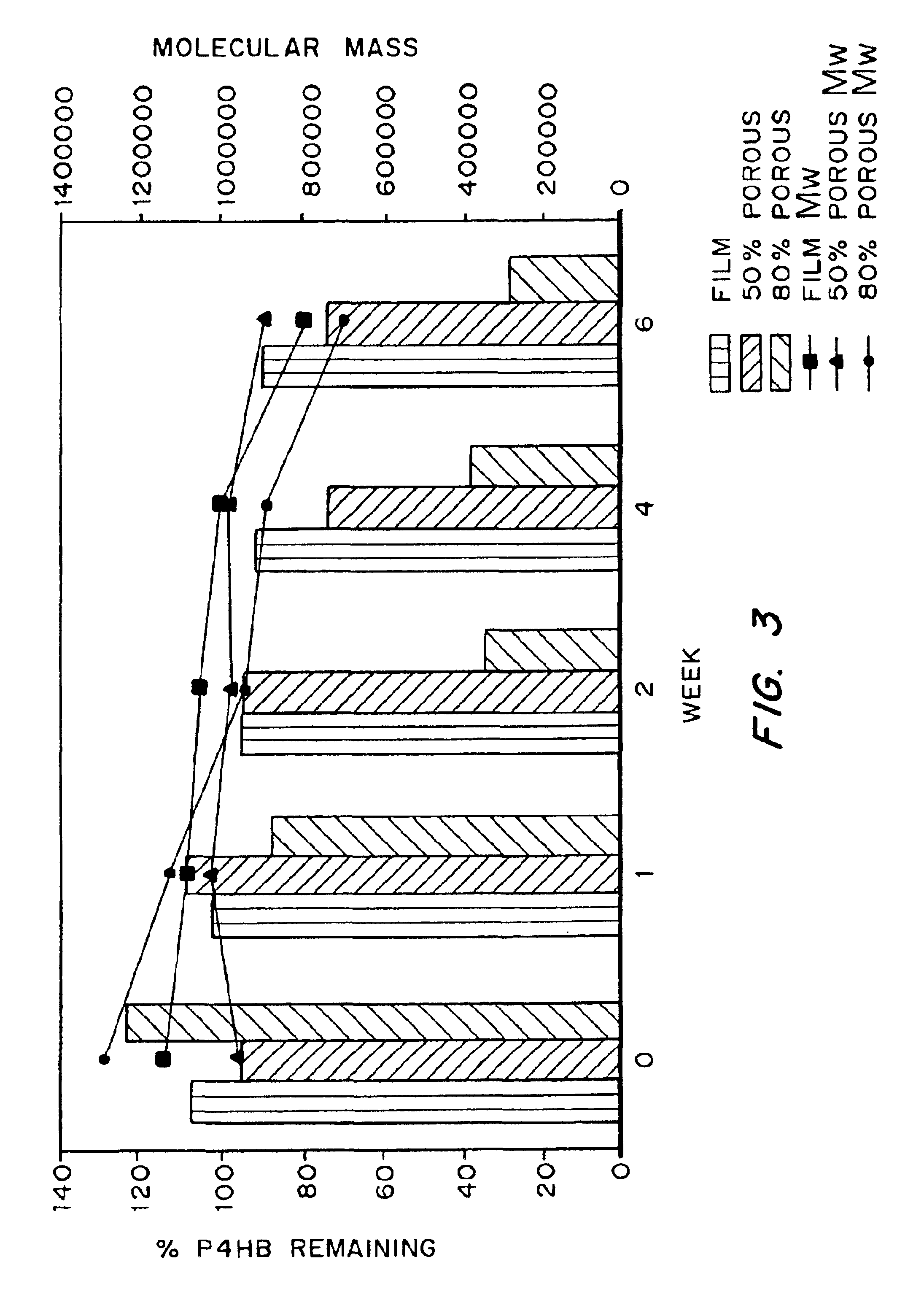
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
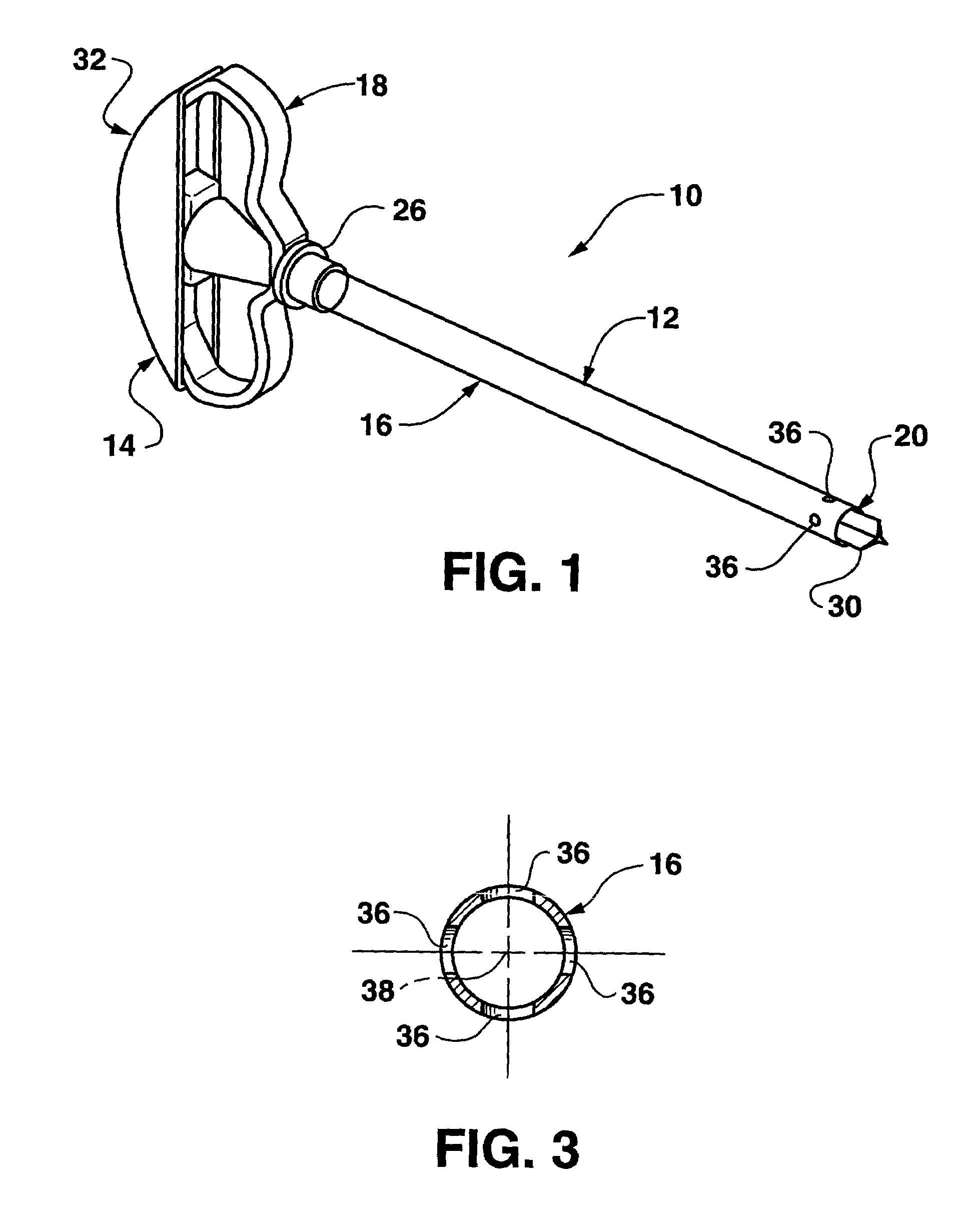
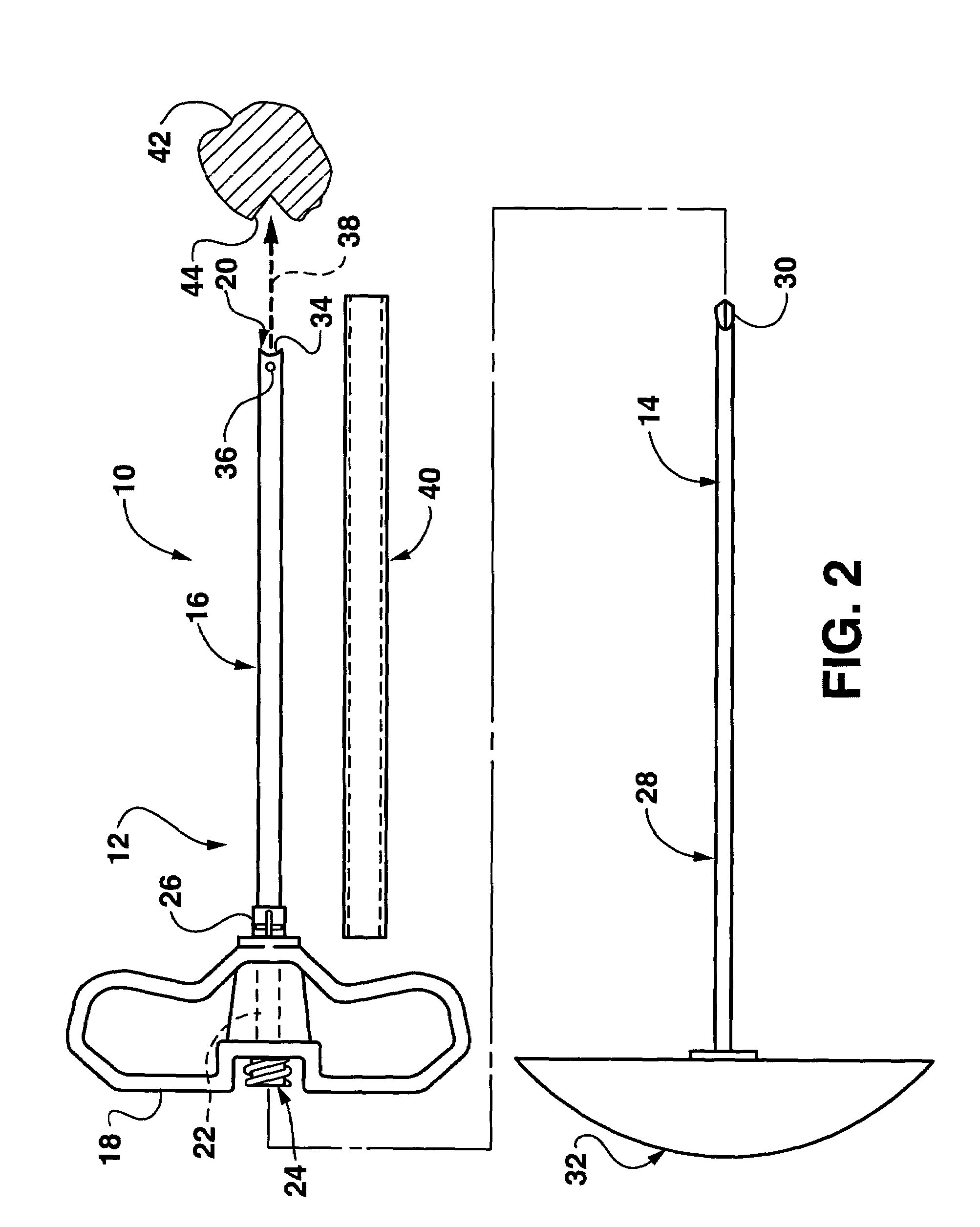
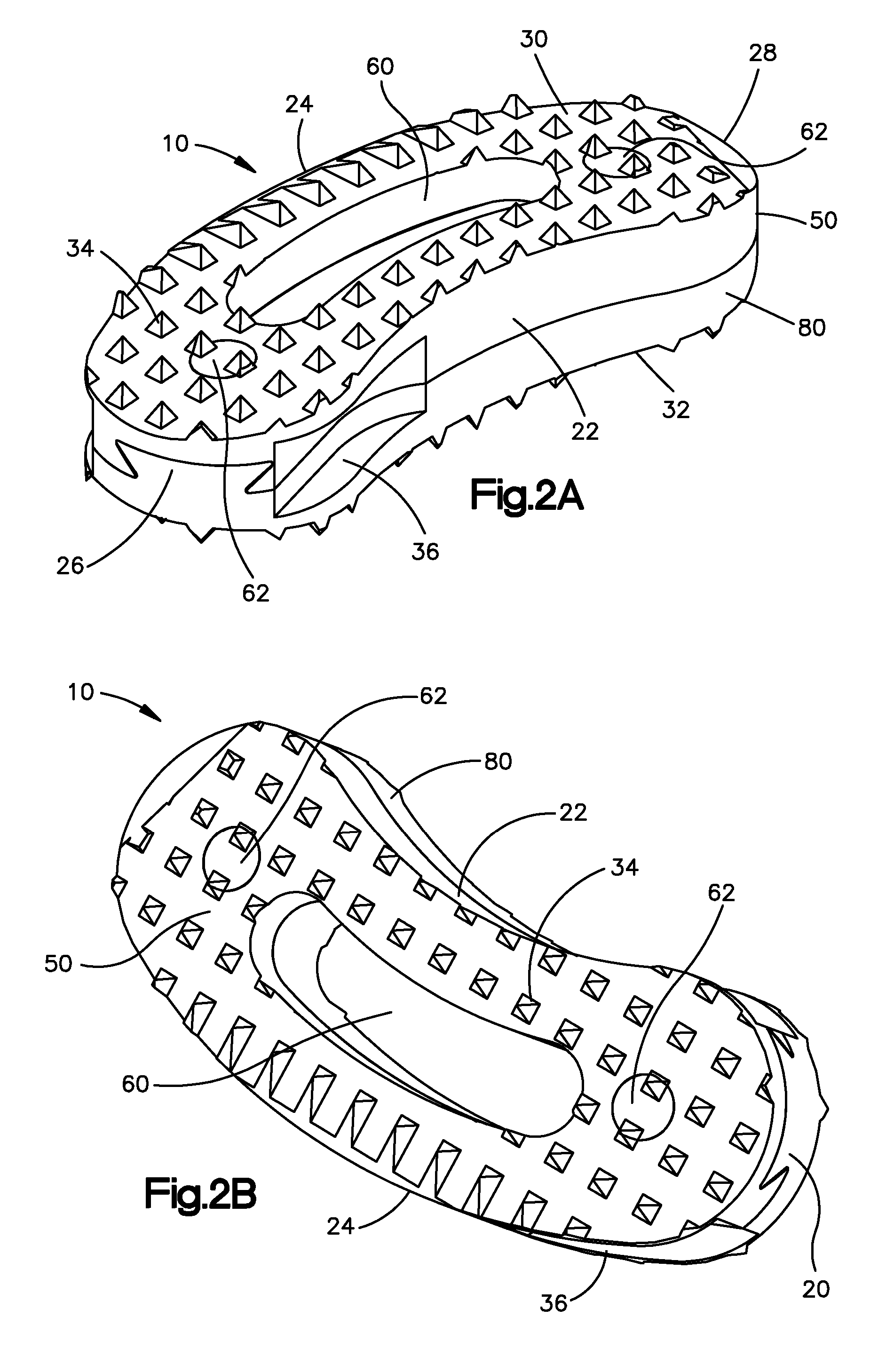
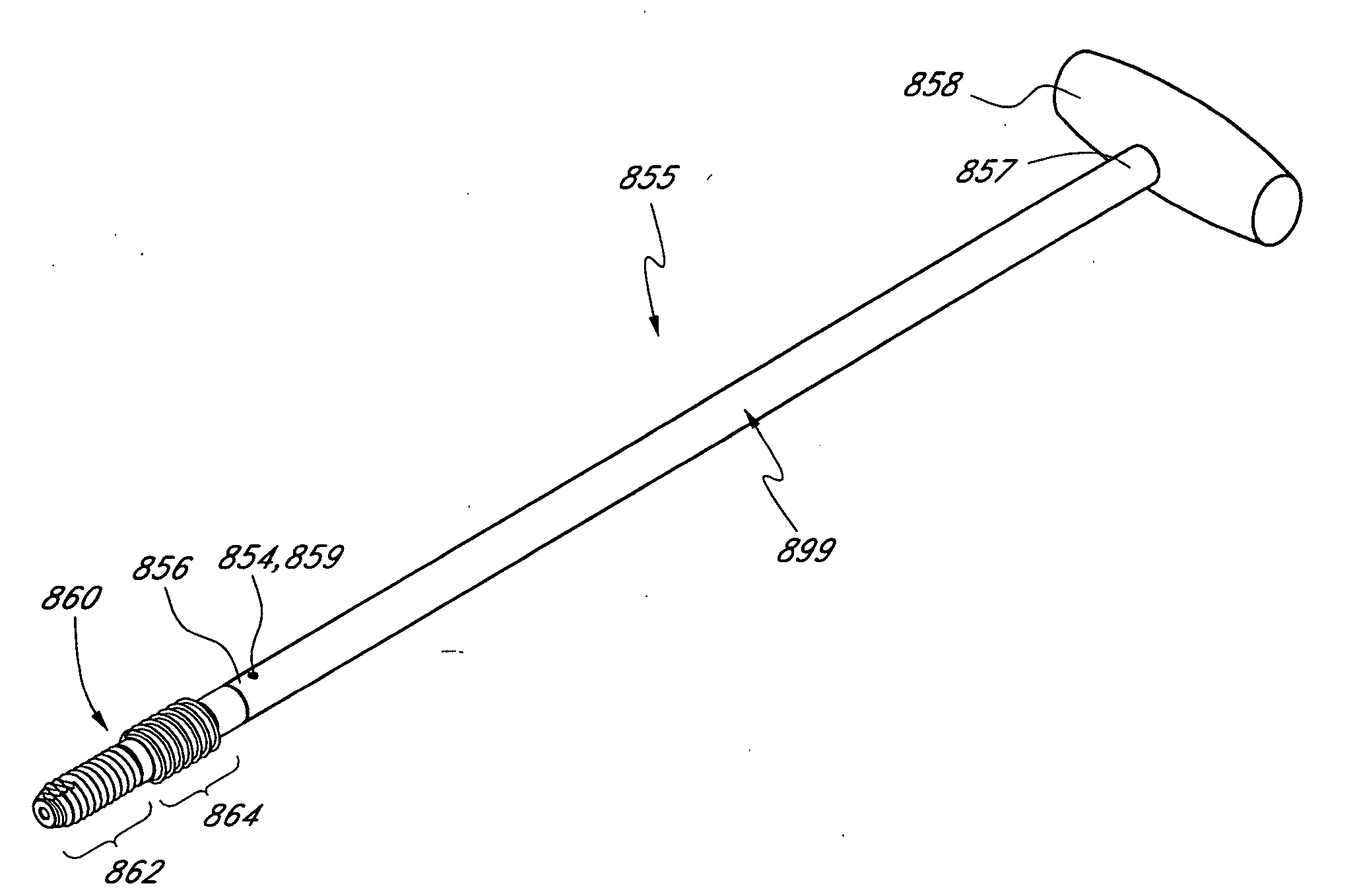
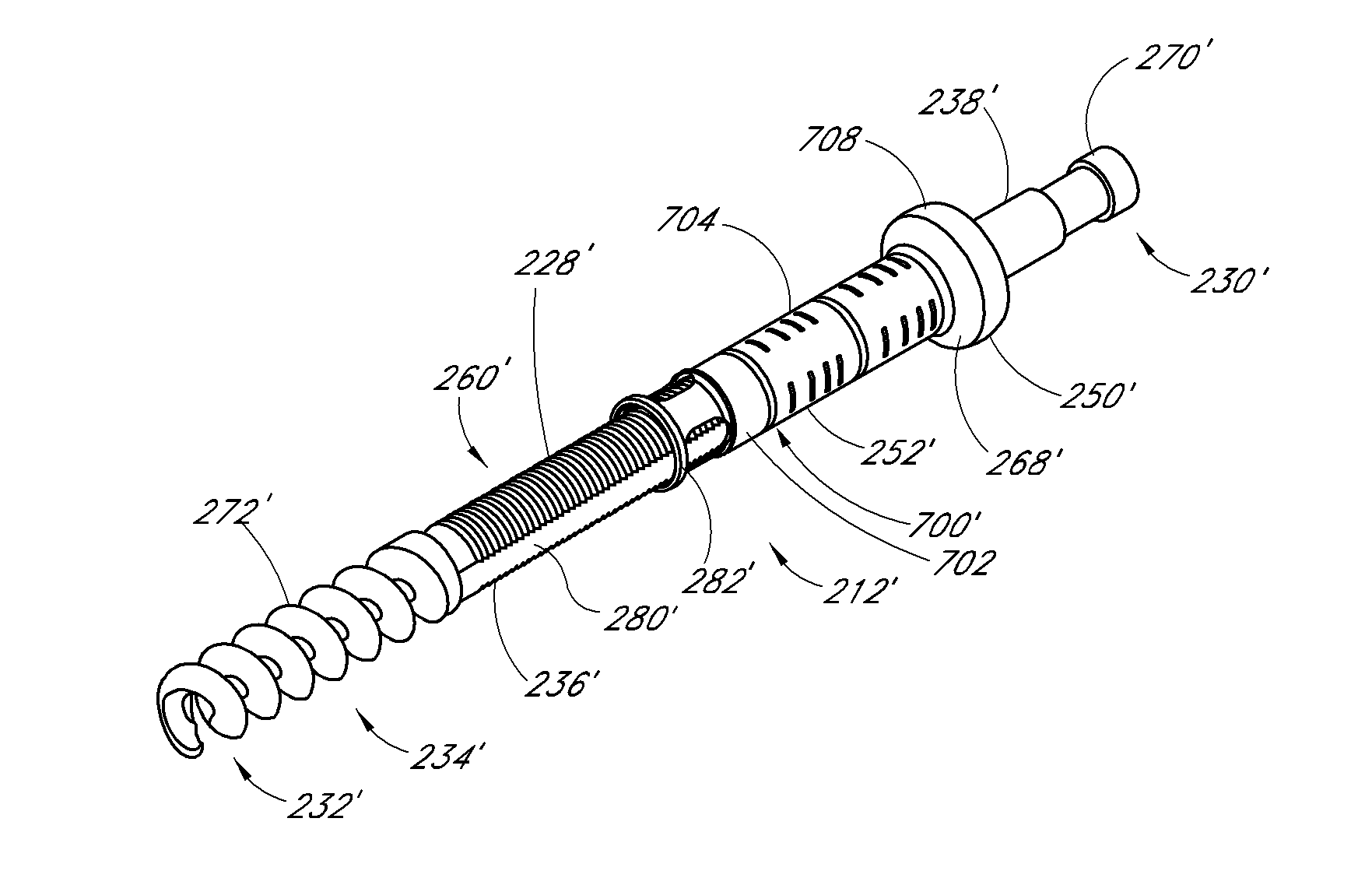
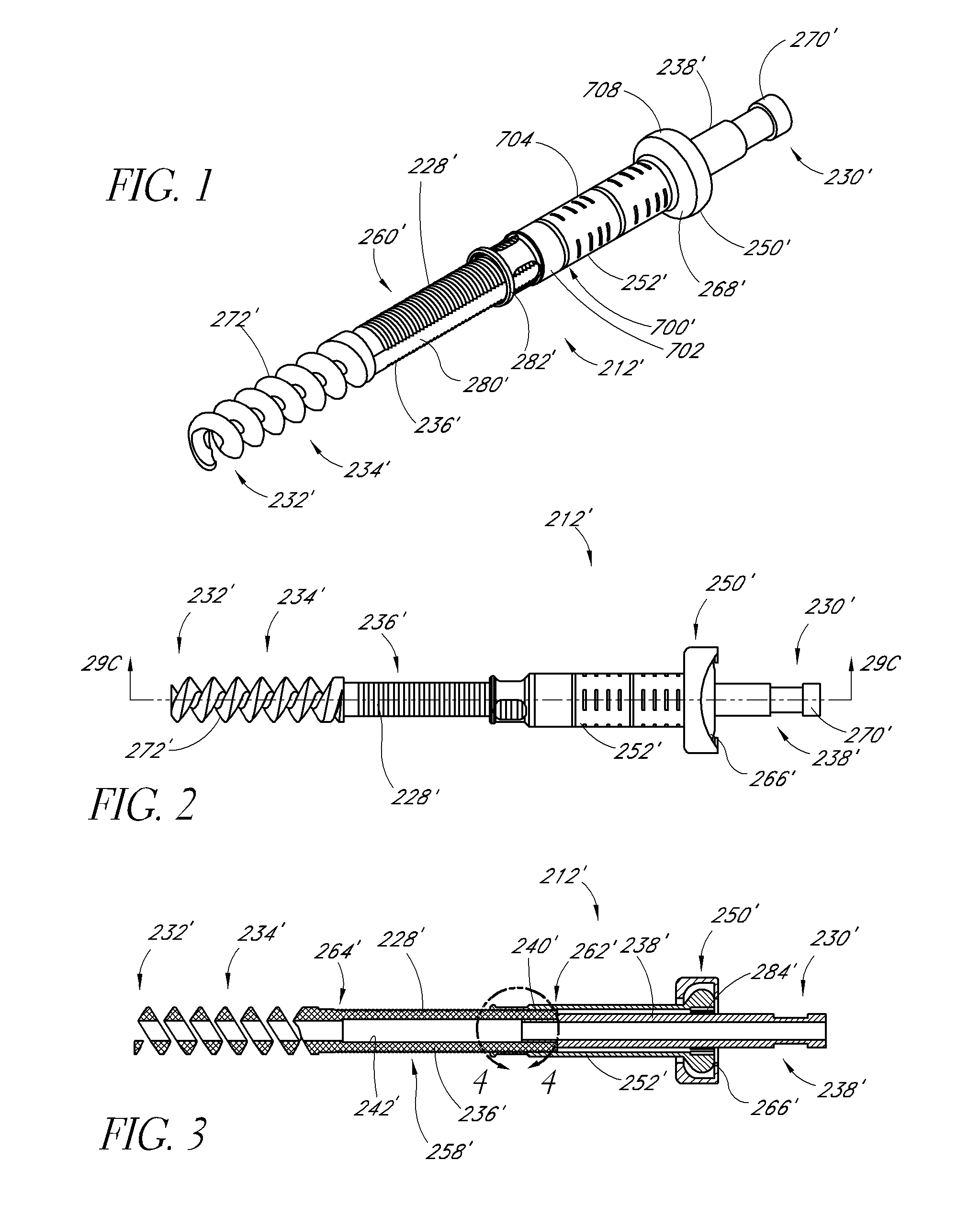
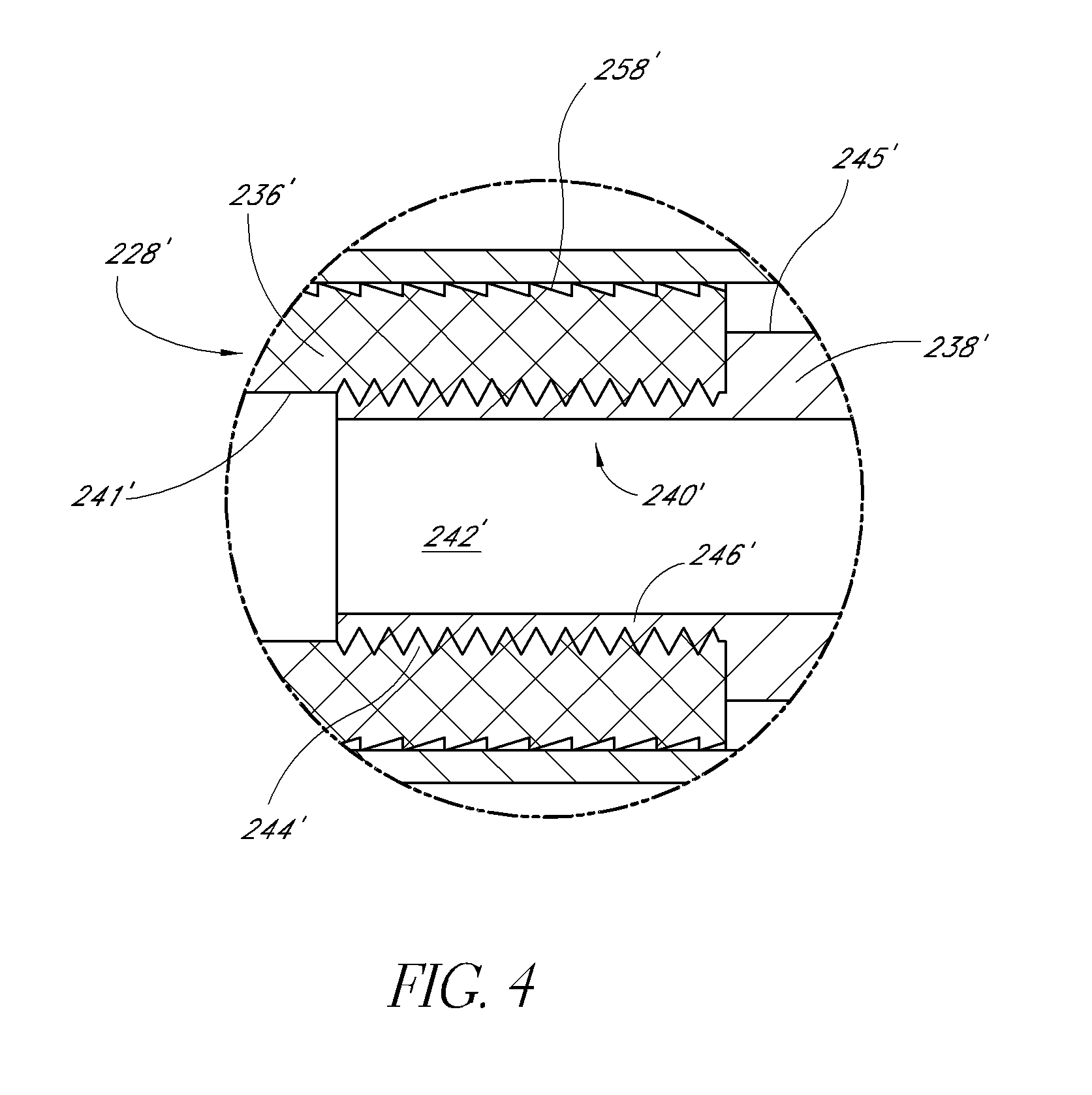
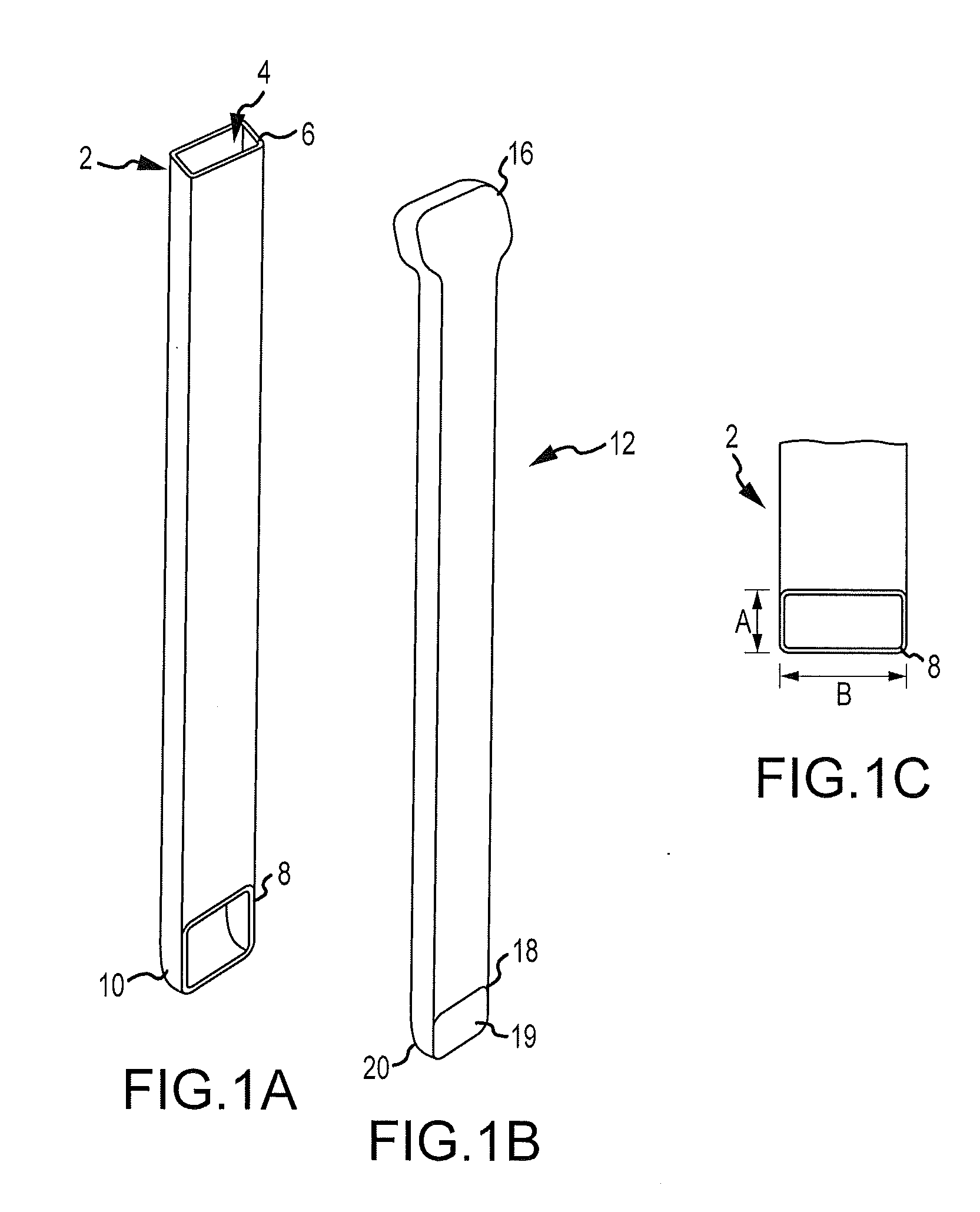
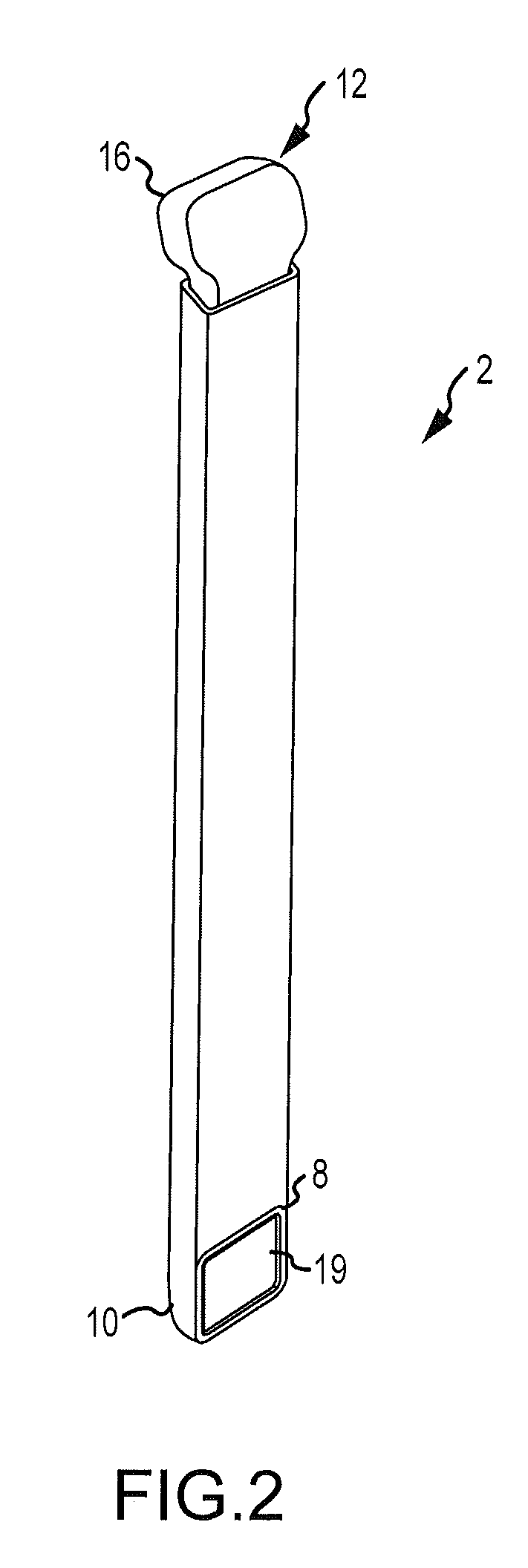
Bendable needle for delivering bone graft material and method of use
ActiveUS7066942B2Surgical needlesVaccination/ovulation diagnosticsMinimally invasive proceduresBone graft materials
A bone graft needle, particularly useful in minimally invasive procedures, is provided. The bone graft needle as well as its corresponding penetrating member may be made from bendable materials so that the combined instrument can more easily access hard to reach areas of the body.
Owner:WRIGHT MEDICAL TECH
Medical and dental implant devices for controlled drug delivery
Implantable devices and methods for use in the treatment of osteonecrosisare provided. The device includes at least one implant device body adapted for insertion into one or more channels or voids in bone tissue; a plurality of discrete reservoirs, which may preferably be microreservoirs, located in the surface of the at least one implant device body; and at least one release system disposed in one or more of the plurality of reservoirs, wherein the release system includes at least one drug selected from the group consisting of bone growth promoters, angiogenesis promoters, analgesics, anesthetics, antibiotics, and combinations thereof. The device body may be formed of a bone graft material, a polymer, a metal, a ceramic, or a combination thereof. The device body may be a monolithic structure, such as one having a cylindrical shape, or it may be in the form of multiple units, such as a plurality of beads.
Owner:MICROCHIPS INC
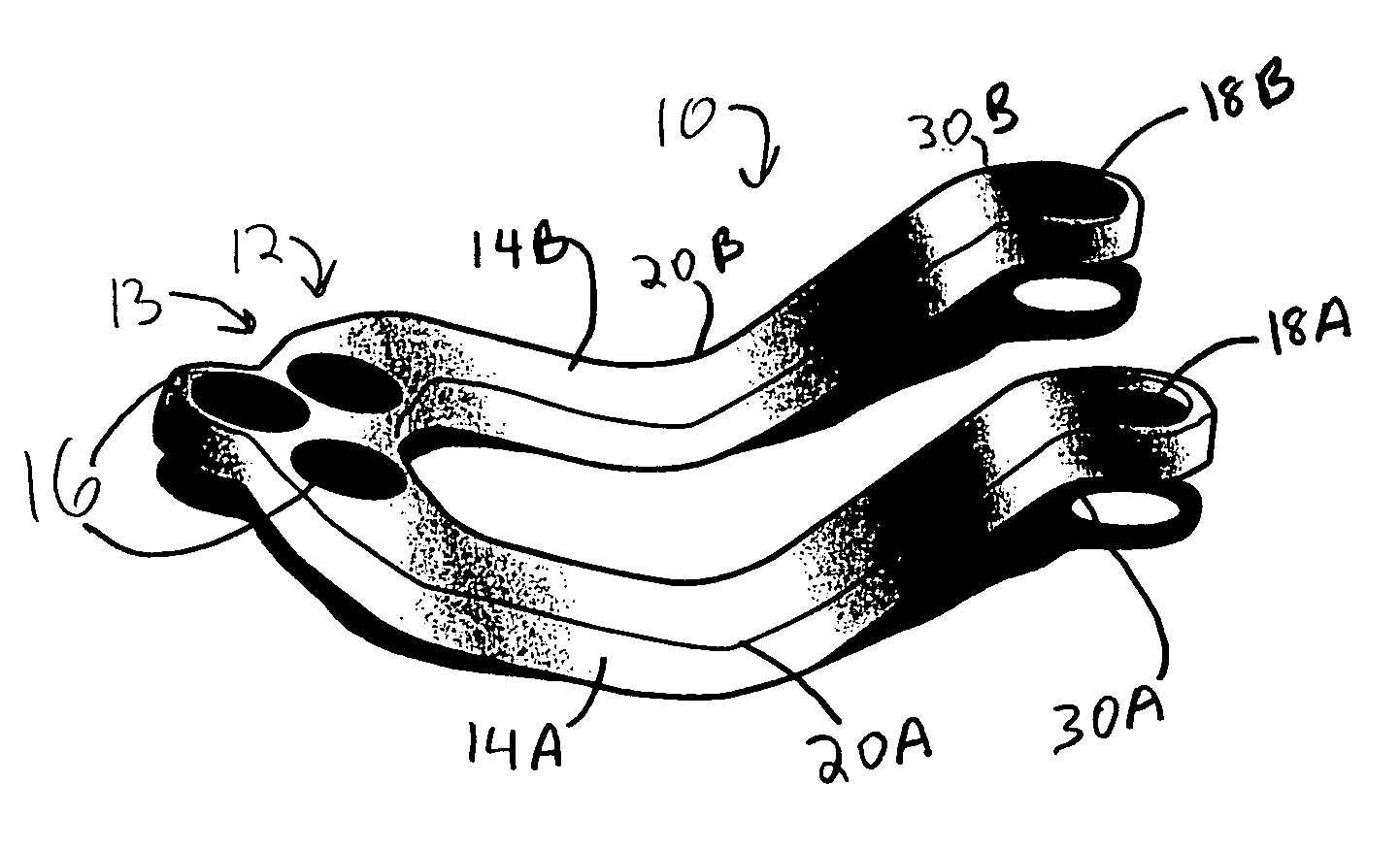
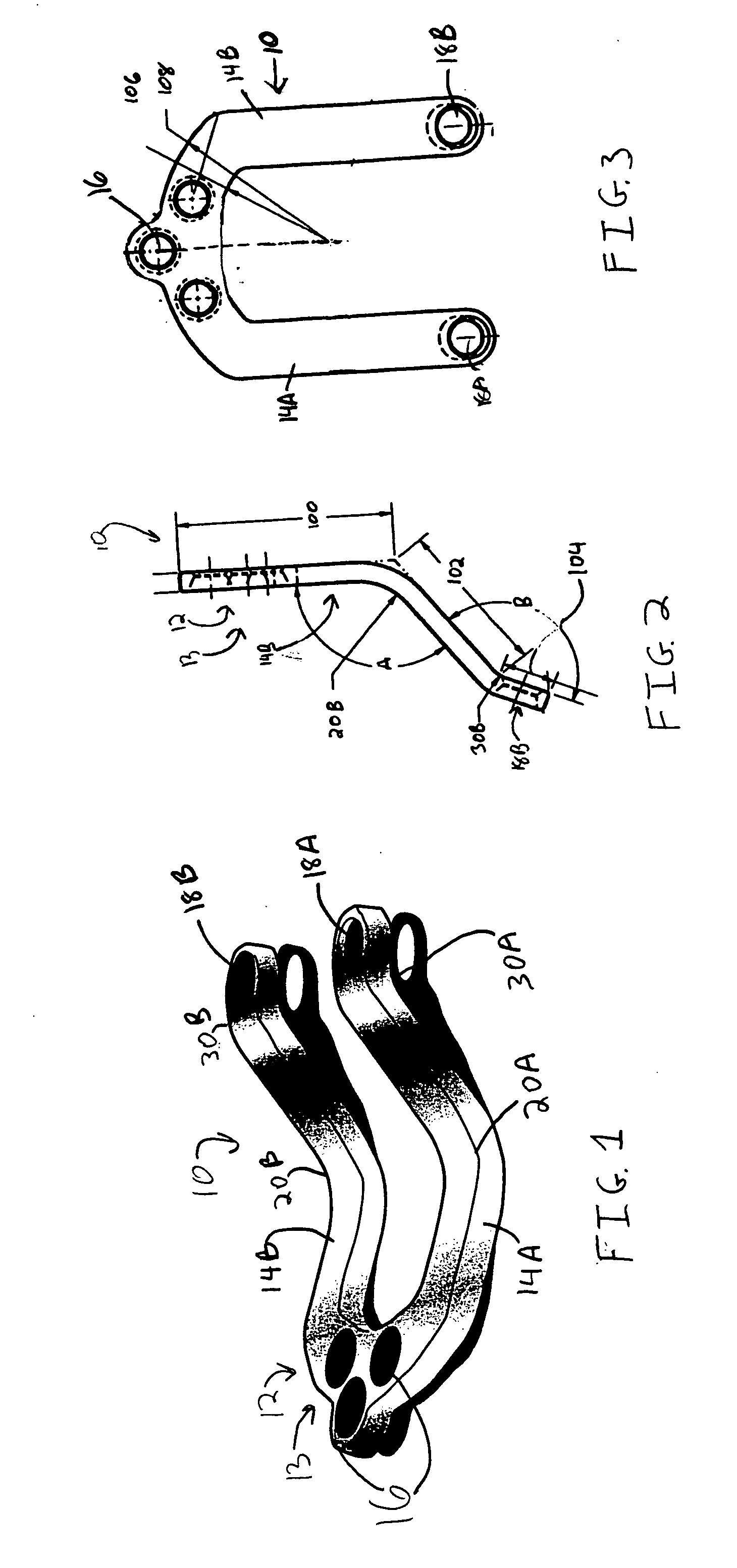
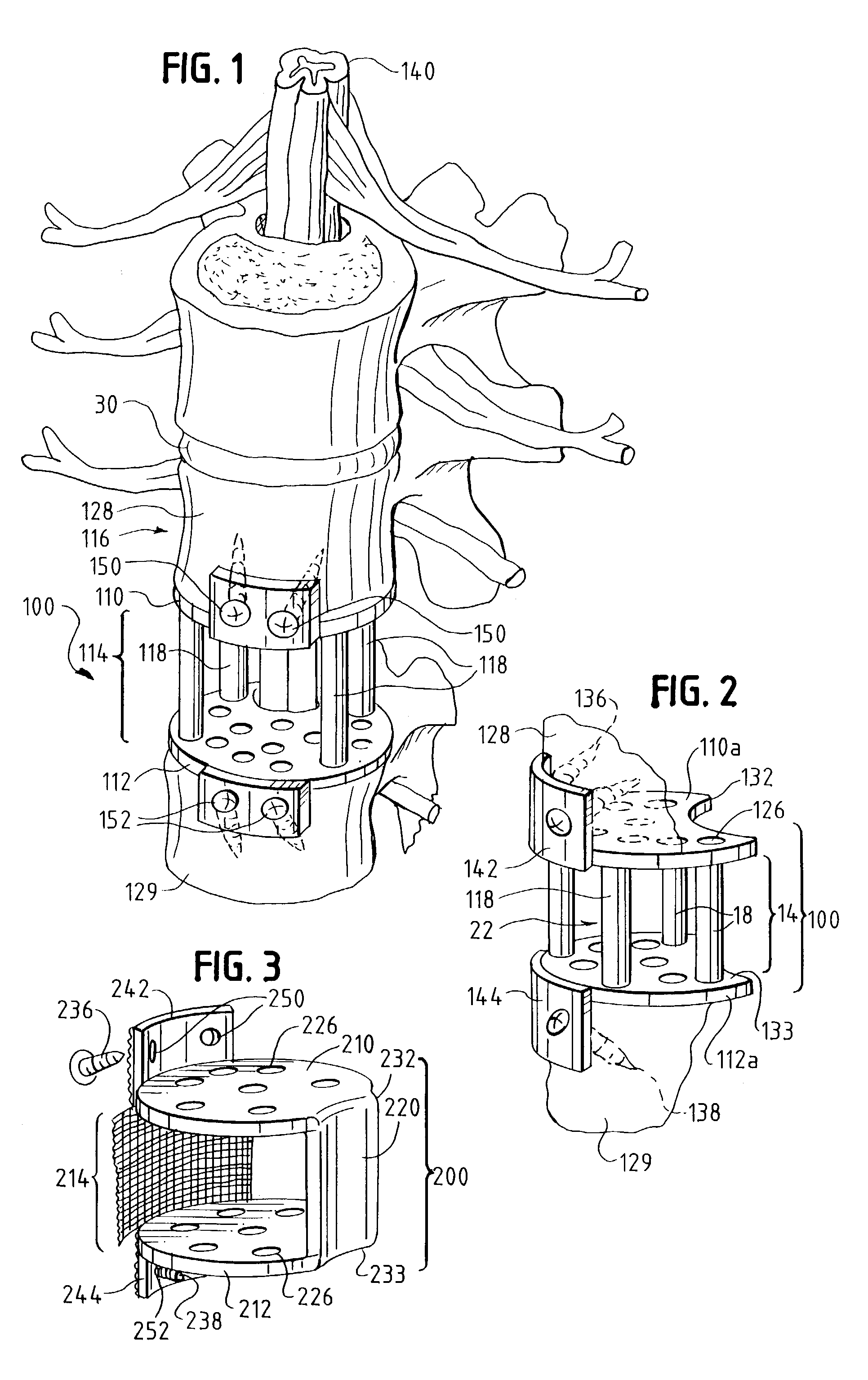
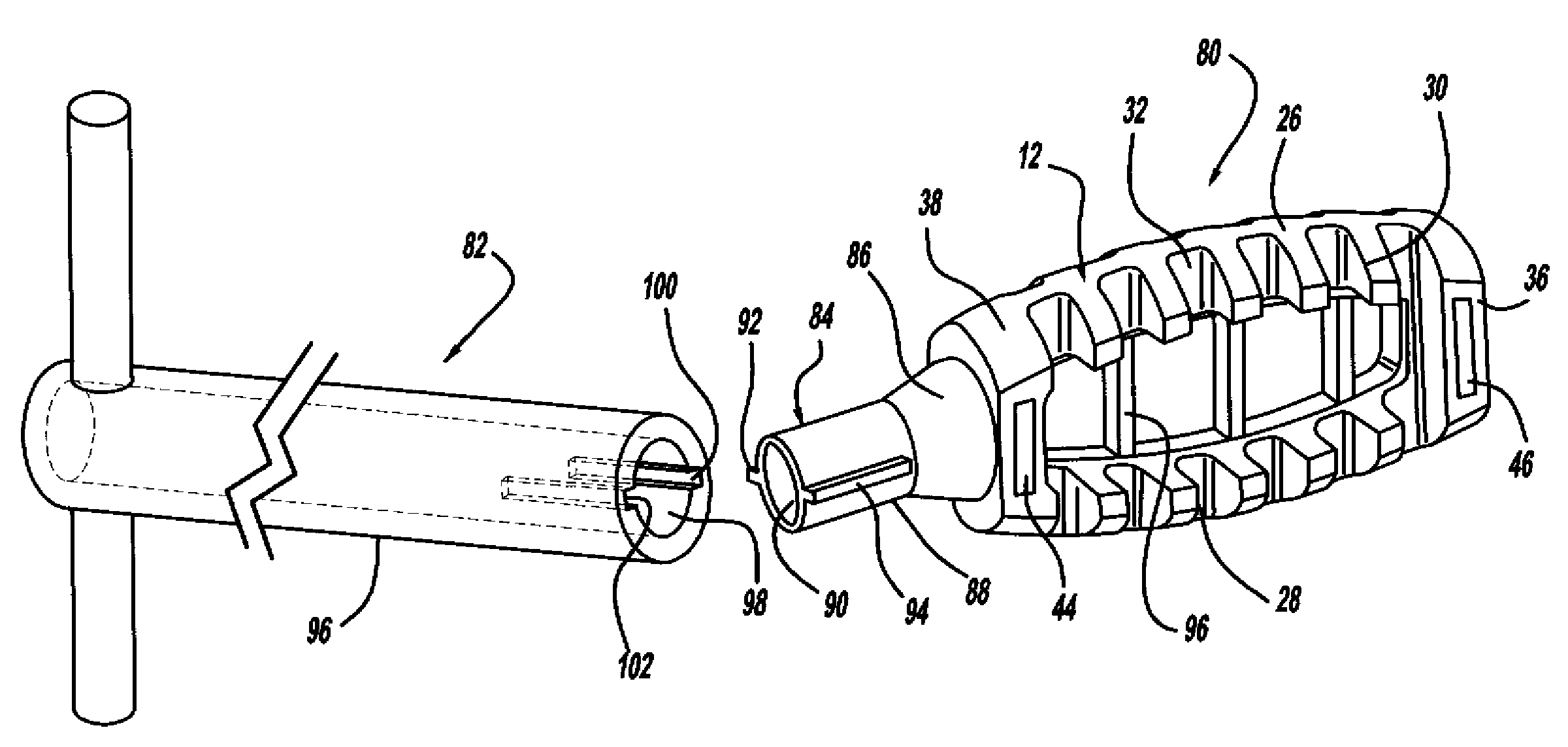
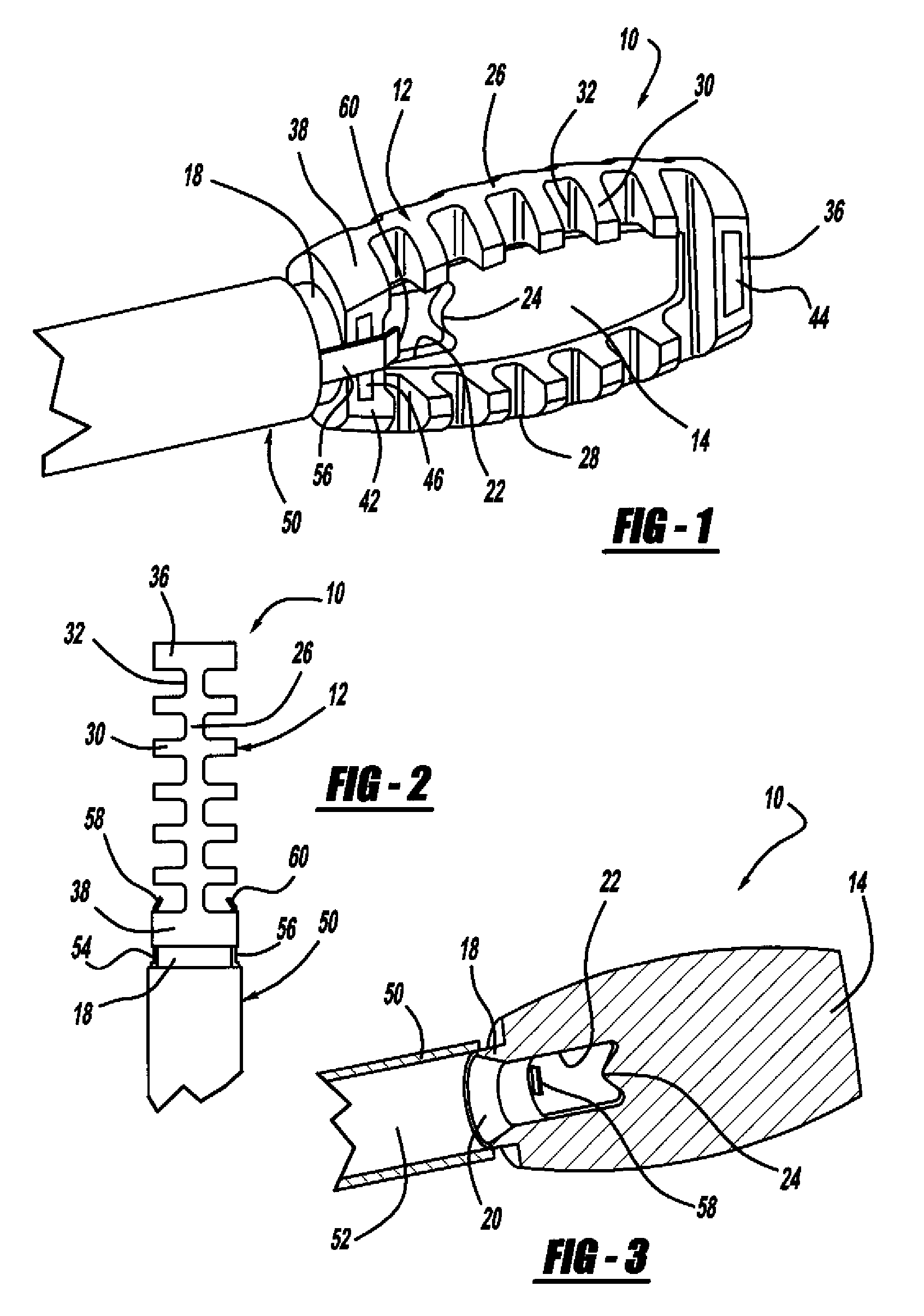
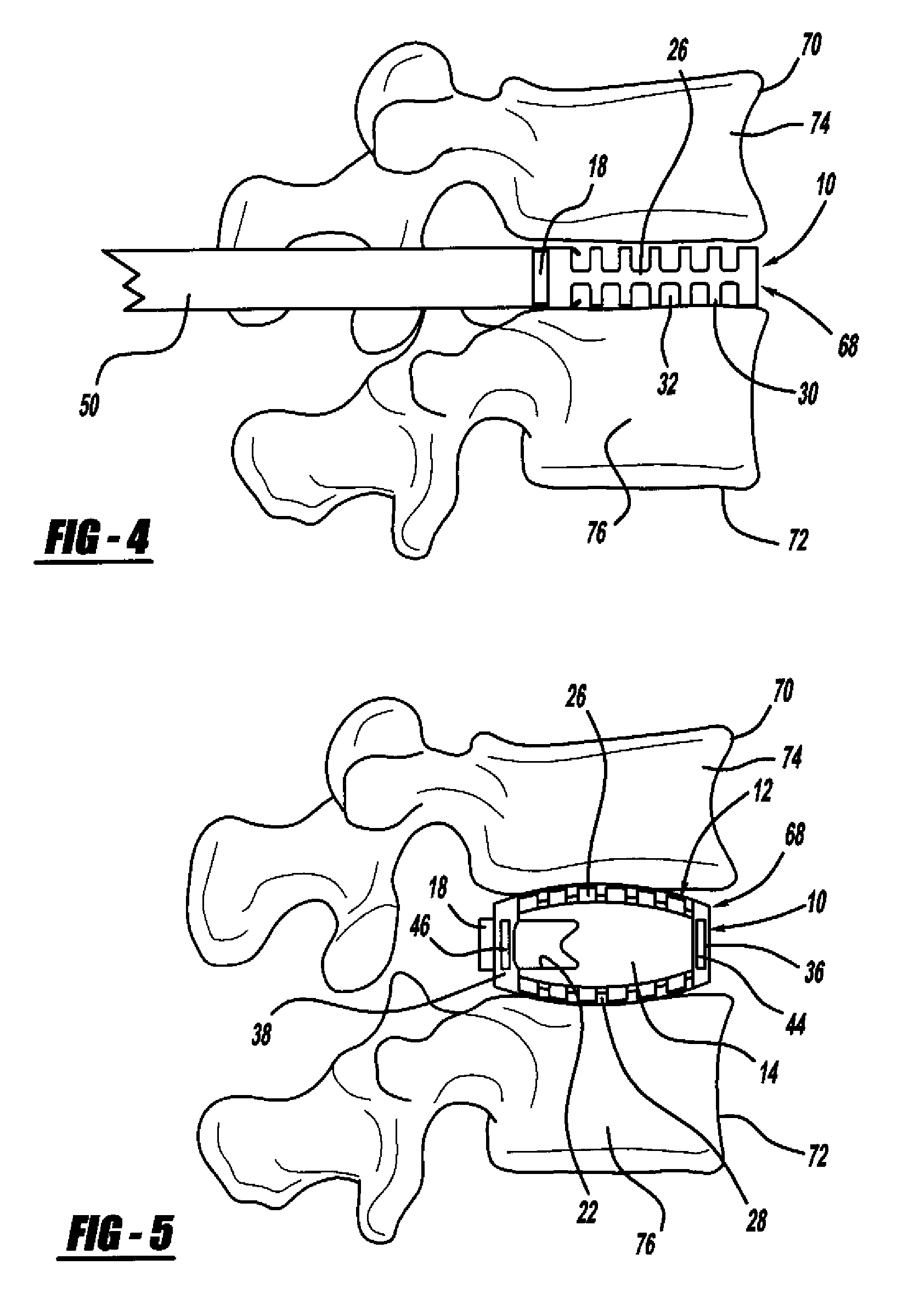
Occipitocervical plate
Methods and systems for occipital-cervical spinal fixation. A plate configured for attachment to the occipital bone has two arms extending out from either side, which turn downwards parallel to one another. A first bend is disposed in the arms, such that the arms extend down from the occipital bone behind the spinous process of the C1 and C2 vertebrae, upon installation. A second bend in the arms allows attachment to the C2 vertebra. The system may be dimensioned for pediatric installation. A bone graft material may be held in place between the cervical vertebrae and the skull by installing a cable to the installed system to retain the bone graft material in place. Methods and kits for occipital-cervical spinal fixation are also disclosed.
Owner:UNIV OF UTAH RES FOUND
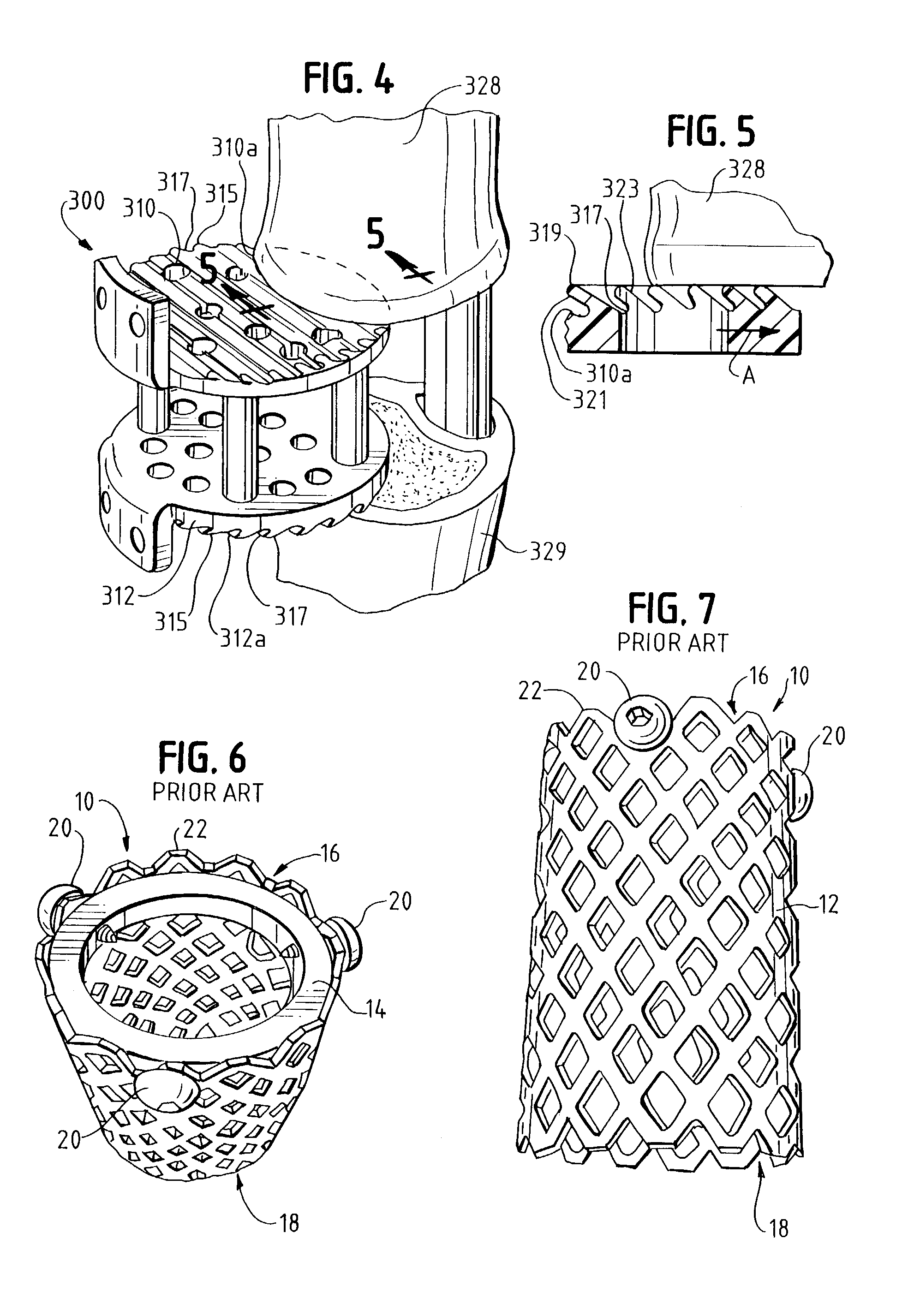
Fusion implant device and method of use
An intervertebral prosthesis includes a bone graft implant member dimensioned for insertion within an intervertebral space defined between adjacent vertebrae and having at least first and second longitudinal sections with respective first and second cross-sectional dimensions. The first cross-sectional dimension of the first implant section is greater than the second cross-section dimension of the second implant section to define a stepped region having a retaining surface. Consequently, upon insertion of the implant member within a generally correspondingly dimensioned receiving bed formed within the adjacent vertebrae, the retaining surface facilitates securement therewithin by corresponding engagement with surfaces of the receiving bed. A method for fusion of adjacent vertebrae utilizing the prosthesis is also disclosed.
Owner:WARSAW ORTHOPEDIC INC
Cervical interbody device
An interbody spacer assembly includes a pair of end pieces spaced apart by a connector extending between them. The end pieces extend generally parallel to the end plates of adjoining vertebral bodies. Fasteners connect the end pieces to the vertebral bodies. Bone graft material or solid bone can be placed in the interior space defined by the end pieces and connector, which bone graft material or solid bone eventually fuses together and to the adjoining end plates through the end pieces.
Owner:WARSAW ORTHOPEDIC INC
Allograft intervertebral implant and method of manufacturing the same
ActiveUS20080082173A1Facilitating spinal fusionBone implantSpinal implantsSacroiliac jointAllograft bone
The present invention is directed to an allograft intervertebral implant sized and configured for insertion between adjacent vertebral bodies in a spinal fusion surgery. The implant is preferably manufactured from two or more pieces of allograft bone joined together by a joint, more preferably a dovetail joint. The dovetail joint being sized and configured to substantially follow the exterior shape or surface (e.g. perimeter) of the intervertebral implant. The intervertebral implant may also include one or more bone pins for joining the allograft pieces, the pins being inserted into the implant at an angle substantially perpendicular with respect to the dovetail joint. The intervertebral implant may also include one or more through-bores for receiving ostegenic or bone graft material. The intervertebral implant is preferably sized and configured for insertion during a T-PLIF or PLIF procedure.
Owner:SYNTHES USA
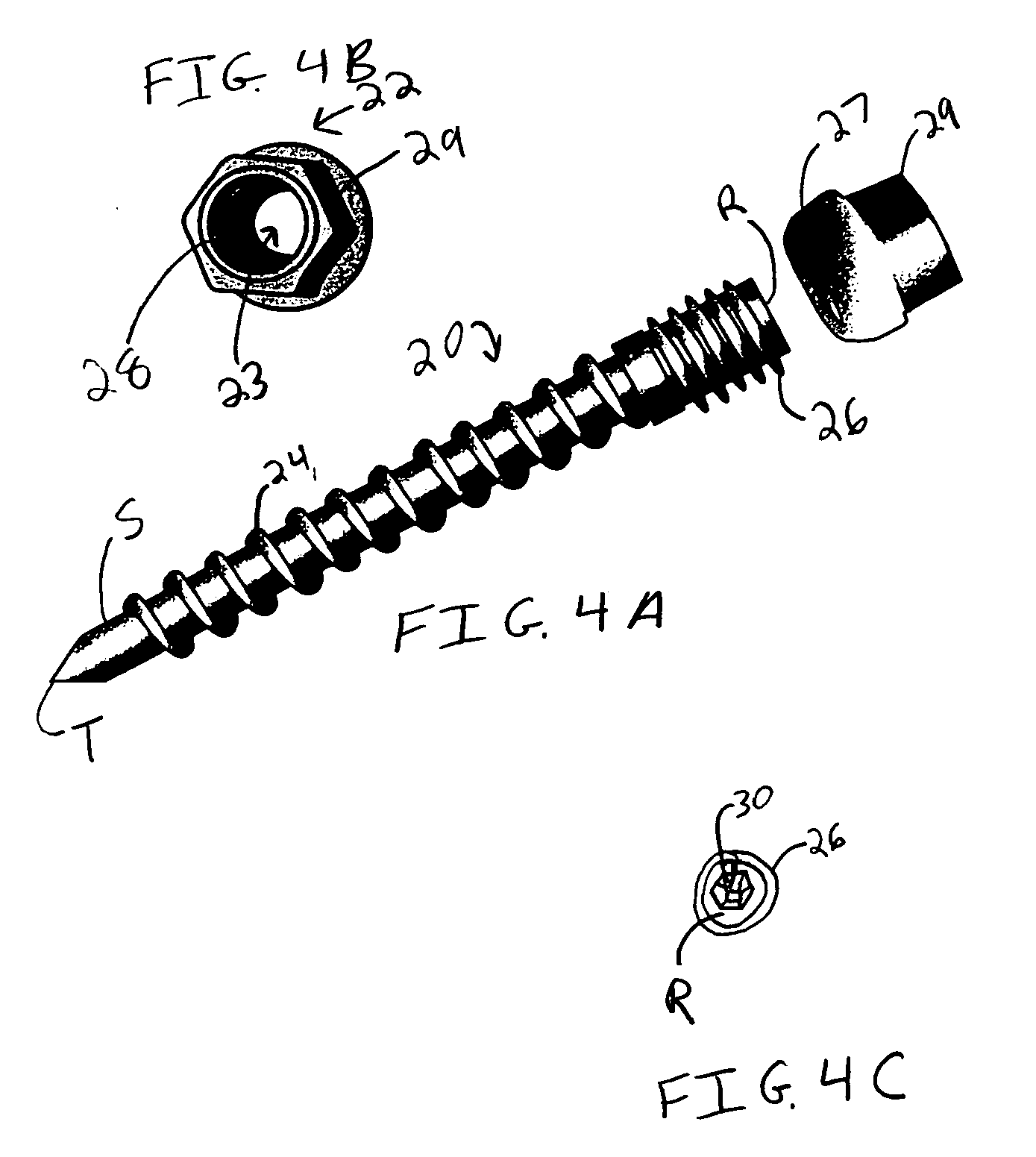
Cervical compression plate assembly
InactiveUS20050043732A1Avoid distractionsLimiting distractionInternal osteosythesisSurgical instrument detailsEngineeringCervical spine
A cervical compression plate assembly having screw receiving elements at opposite ends that are configured for engaging bone fixation screws extending from respective vertebral elements. The assembly permits the distance between the screw receiving elements at opposite ends to be shortened but prevents the distance from increasing. A spring mechanism is housed within the plate assembly and is configured for continuously urging the screw receiving elements at opposite ends together for thereby providing continuous compressive loading on bone graft material disposed between the vertebral elements.
Owner:DALTON BRIAN E
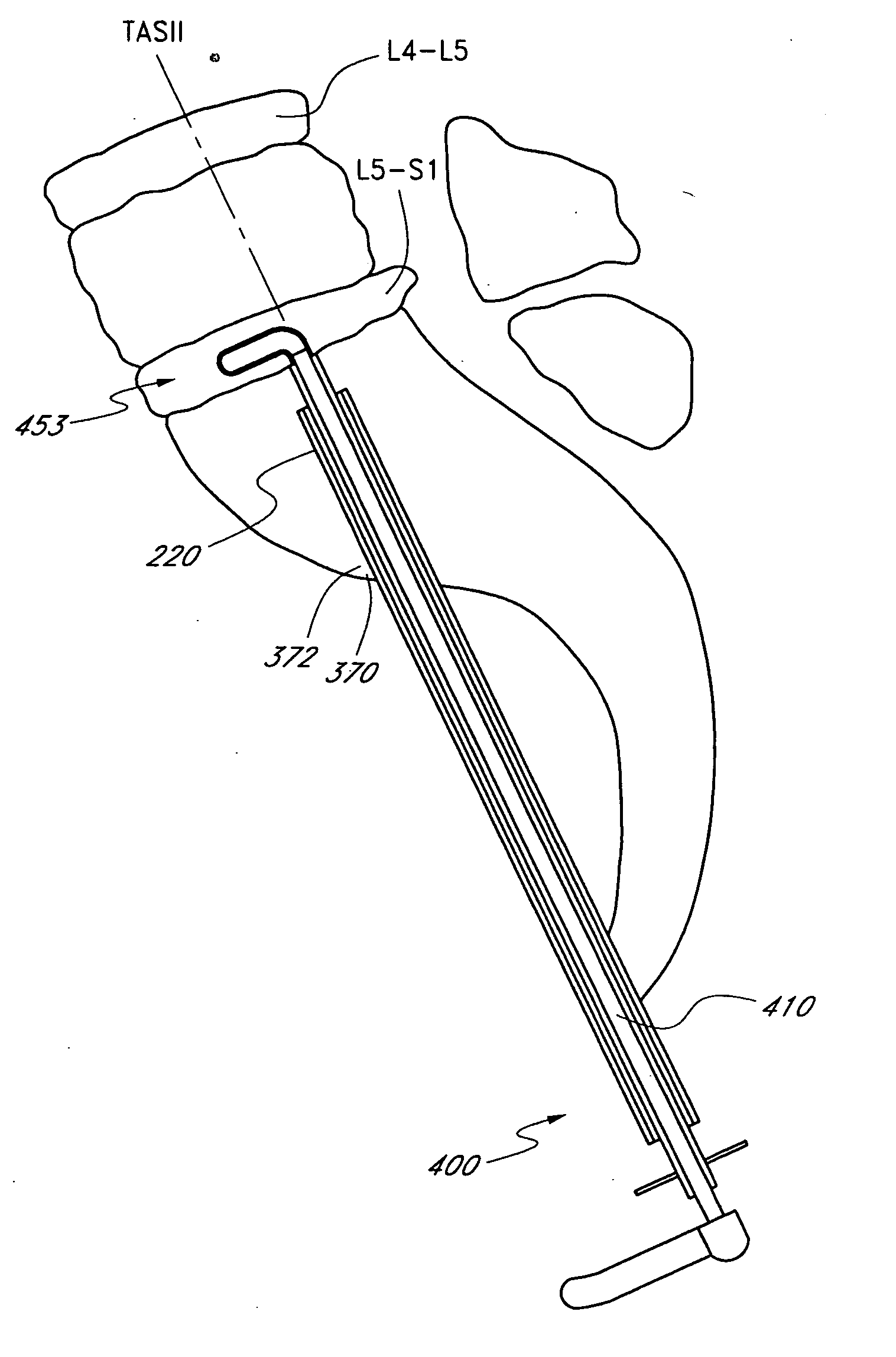
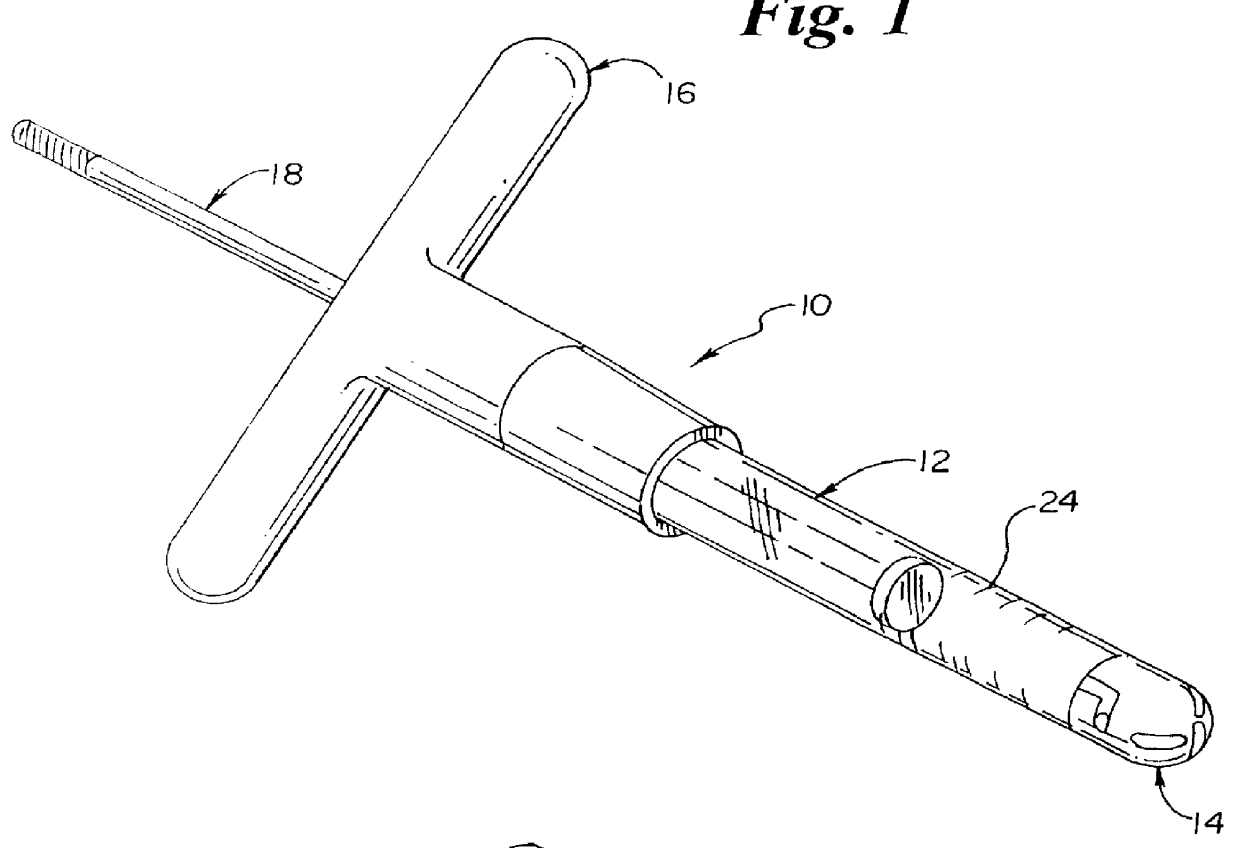
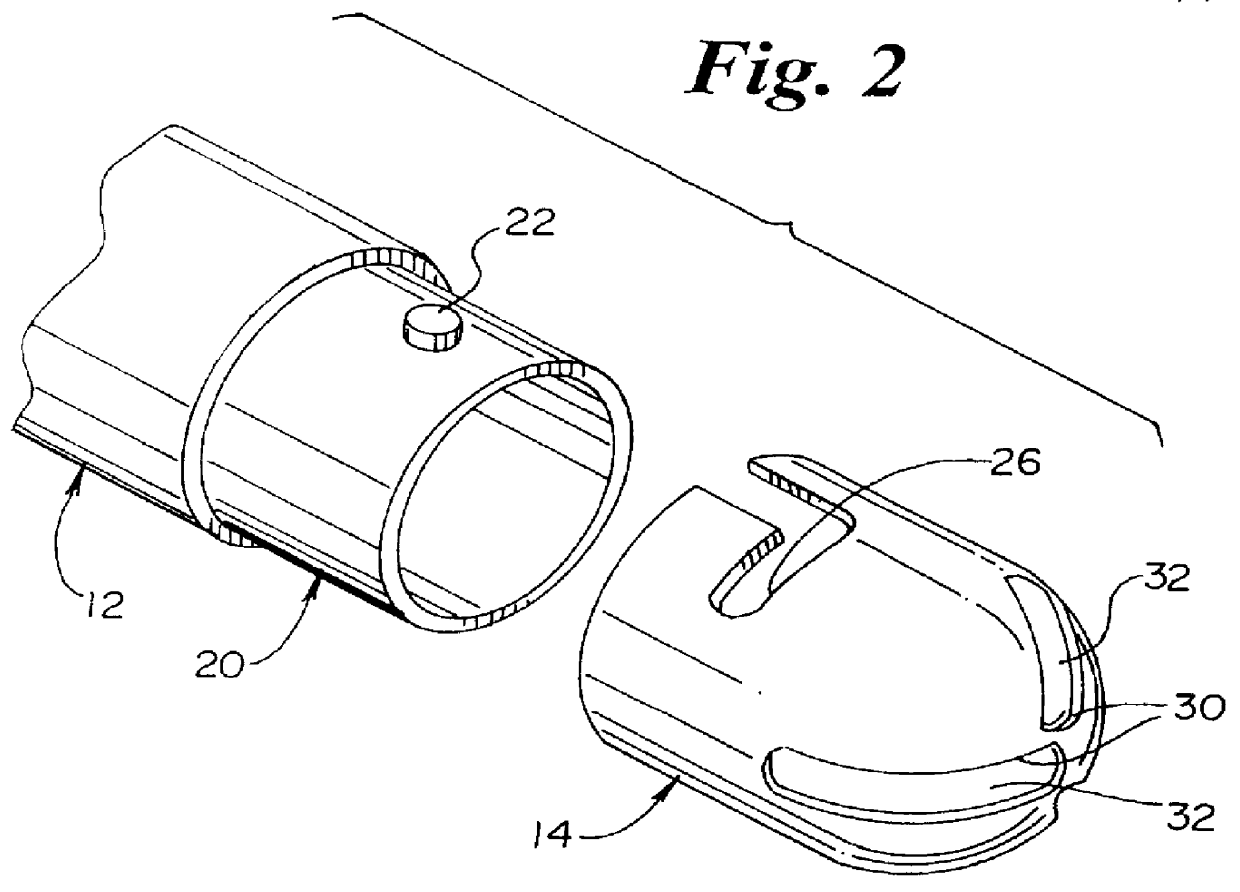
Method and apparatus for spinal distraction
ActiveUS20050137602A1Less immediate traumaConvenient amountInternal osteosythesisCannulasDistractionSpinal column
Disclosed are surgical tools, tool sets and methods for percutaneously accessing and preparing treatment sites within the spine for subsequent treatment procedures. The treatment site may be an inter-vertebral motion segments in the lumbar and sacral regions of the spine. The tool set may comprise introducer tools and bone dilators for accessing and tapping into a targeted site, such as, for example, the anterior surface of the S1 vertebral body. The tool set may also comprise cutters and extractors for preparing the treatment site for subsequent treatment procedures. The tool set may additionally comprise a bone graft inserter, an exchange system, and / or a temporary distraction tool for further preparing the treatment site for subsequent treatment procedures.
Owner:MIS IP HLDG LLC
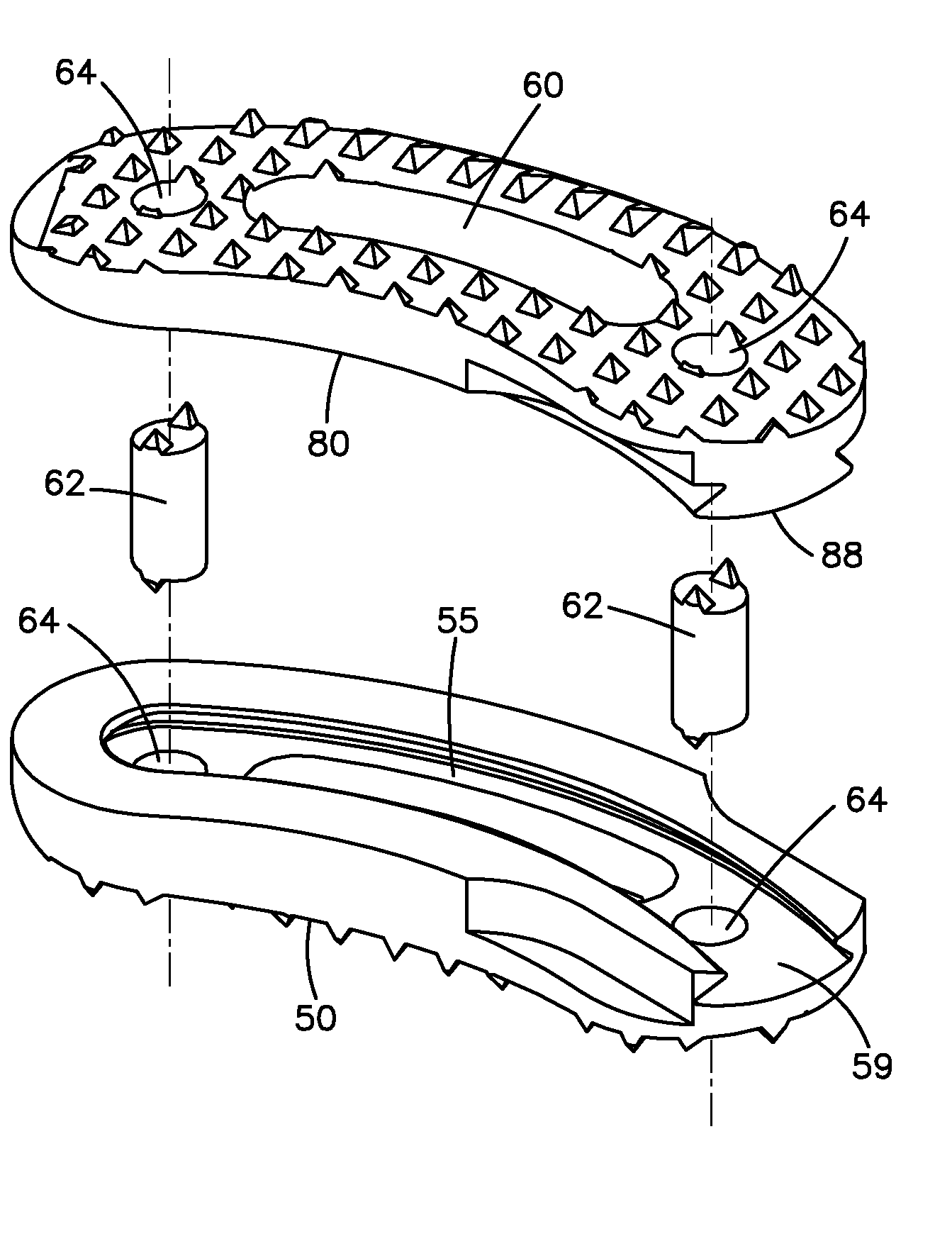
Selectively Expanding Spine Cage With Enhanced Bone Graft Infusion
A selectively expanding spine cage has a minimized cross section in its unexpanded state that is smaller than the diameter of the neuroforamen through which it passes in the distracted spine. The cage conformably engages between the endplates of the adjacent vertebrae to effectively distract the anterior disc space, stabilize the motion segments and eliminate pathologic spine motion. Expanding selectively (anteriorly, along the vertical axis of the spine) rather than uniformly, the cage height increases and holds the vertebrae with fixation forces greater than adjacent bone and soft tissue failure forces in natural lordosis. Stability is thus achieved immediately, enabling patient function by eliminating painful motion. The cage shape intends to rest proximate to the anterior column cortices securing the desired spread and fixation, allowing for bone graft in, around, and through the implant for arthrodesis whereas for arthroplasty it fixes to endpoints but cushions the spine naturally.
Owner:HOWMEDICA OSTEONICS CORP
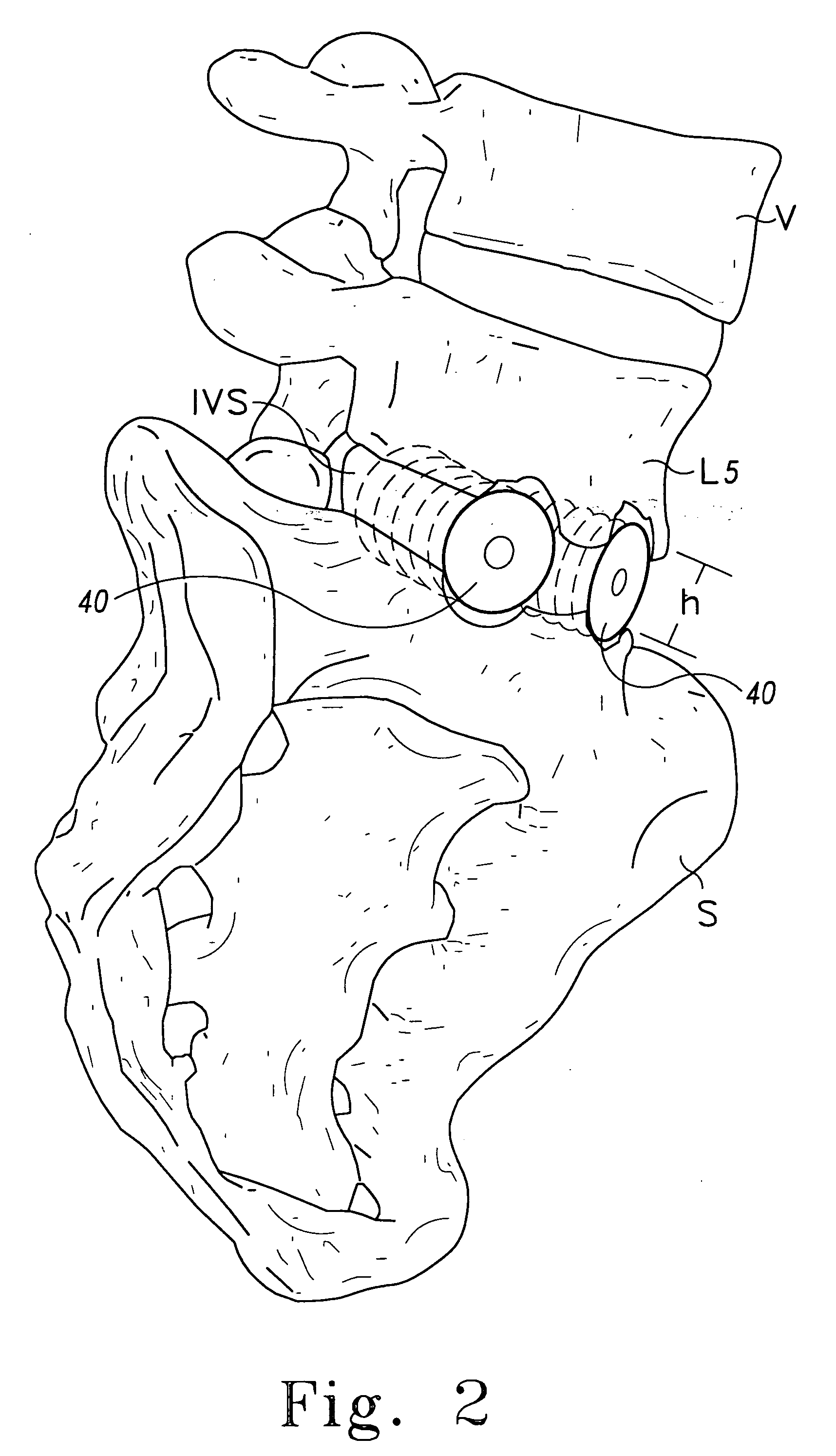
Bone graft
ActiveUS7163691B2Fast reduction in osteoinductiveGood curative effectOrganic active ingredientsImpression capsOSTEOINDUCTIVE FACTORIn vivo
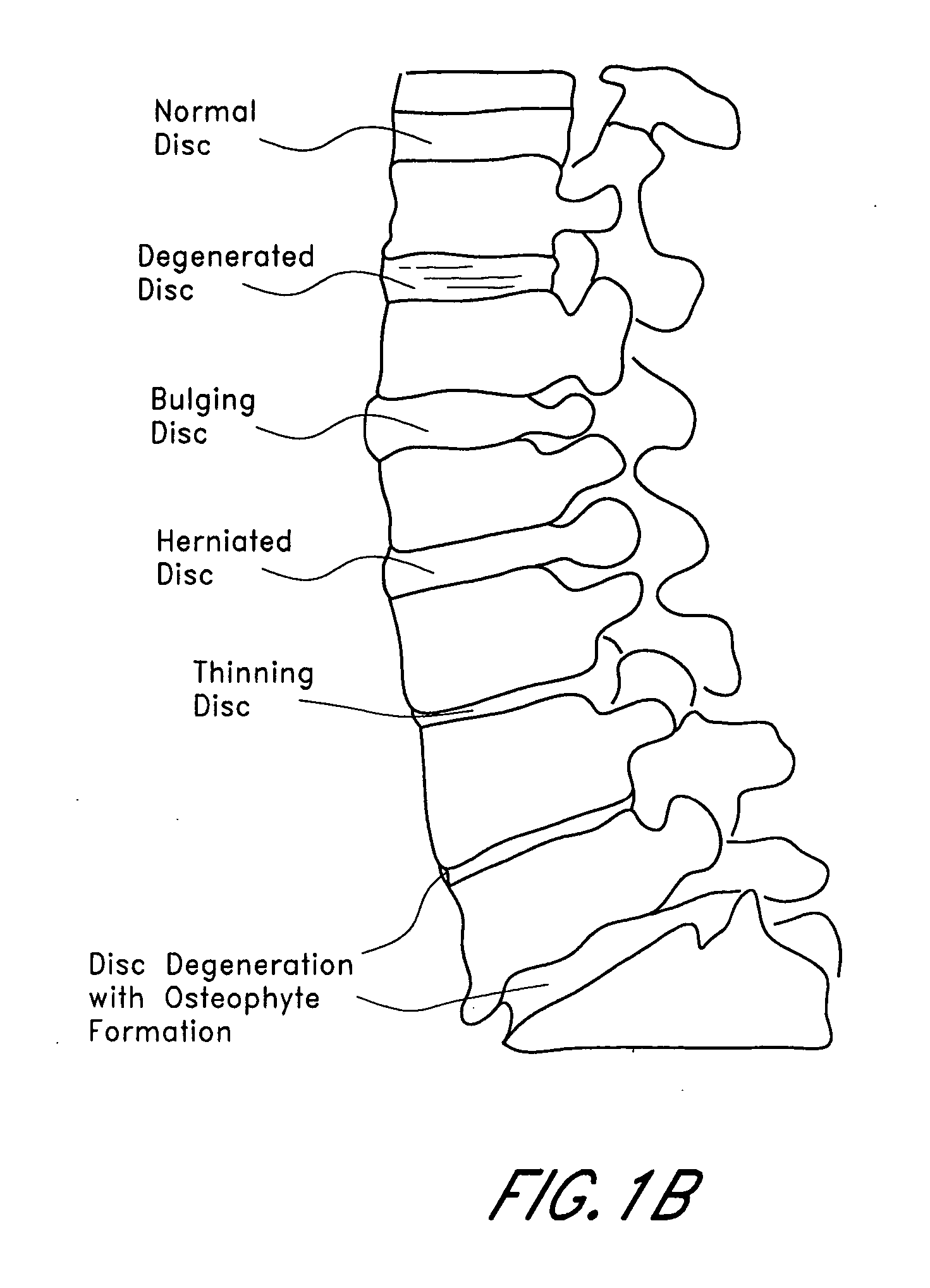
An improved demineralized bone matrix (DBM) or other matrix composition is provided that has been mixed with a stabilizing agent that acts as (1) a diffusion barrier, (2) a enzyme inhibitor, (3) a competitive substrate, or (4) a masking moiety. A diffusion barrier acts as a barrier so as to protect the osteoinductive factors found in DBM from being degraded by proteolytic and glycolytic enzymes at the implantation site. Stabilizing agents may be any biodegradable material such as starches, modified starches, cellulose, dextran, polymers, proteins, and collagen. As the stabilizing agents degrades or dissolves in vivo, the osteoinductive factors such as TGF-β, BMP, and IGF are activated or exposed, and the activated factors work to recruit cells from the preivascular space to the site of injury and to cause differentiation into bone-forming cells. The invention also provides methods of preparing, testing, and using the inventive improved osteodinductive matrix compositions.
Owner:WARSAW ORTHOPEDIC INC
System and method for spinal fixation
InactiveUS20100331891A1Increase ratingsQuality of the joint formed between fixated bones can be improvedSuture equipmentsInternal osteosythesisSpinal columnDilator
A system and method of bone fixation are provided for improving the bone growth and stability of the fixated bones. For example, a target site for a bone fixation procedure can be accessed at a facet of a first vertebra using a tissue dilator. Bone material can be disrupting from or at the target site, and a bone fixation device can be installed to fix the first vertebra relative to a second vertebra. The disruption and / or removal of the bone material, such as by rasping facets or a facet joint of the first vertebra and the second vertebra, can tend to promote bone growth. Further, it is contemplated that bone graft material can be inserted at the target site, such as into a joint space formed between facets of the first vertebra and the second vertebra.
Owner:INTERVENTIONAL SPINE
Method and apparatus for manipulating material in the spine
InactiveUS20050149034A1Less immediate traumaConvenient amountInternal osteosythesisCannulasTreatments proceduresAnterior surface
Disclosed are surgical tools, tool sets and methods for percutaneously accessing and preparing treatment sites within the spine for subsequent treatment procedures. The treatment site may be an inter-vertebral motion segments in the lumbar and sacral regions of the spine. The tool set may comprise introducer tools and bone dilators for accessing and tapping into a targeted site, such as, for example, the anterior surface of the S1 vertebral body. The tool set may also comprise cutters and extractors for preparing the treatment site for subsequent treatment procedures. The tool set may additionally comprise a bone graft inserter, an exchange system, and / or a temporary distraction tool for further preparing the treatment site for subsequent treatment procedures.
Owner:MIS IP HLDG LLC
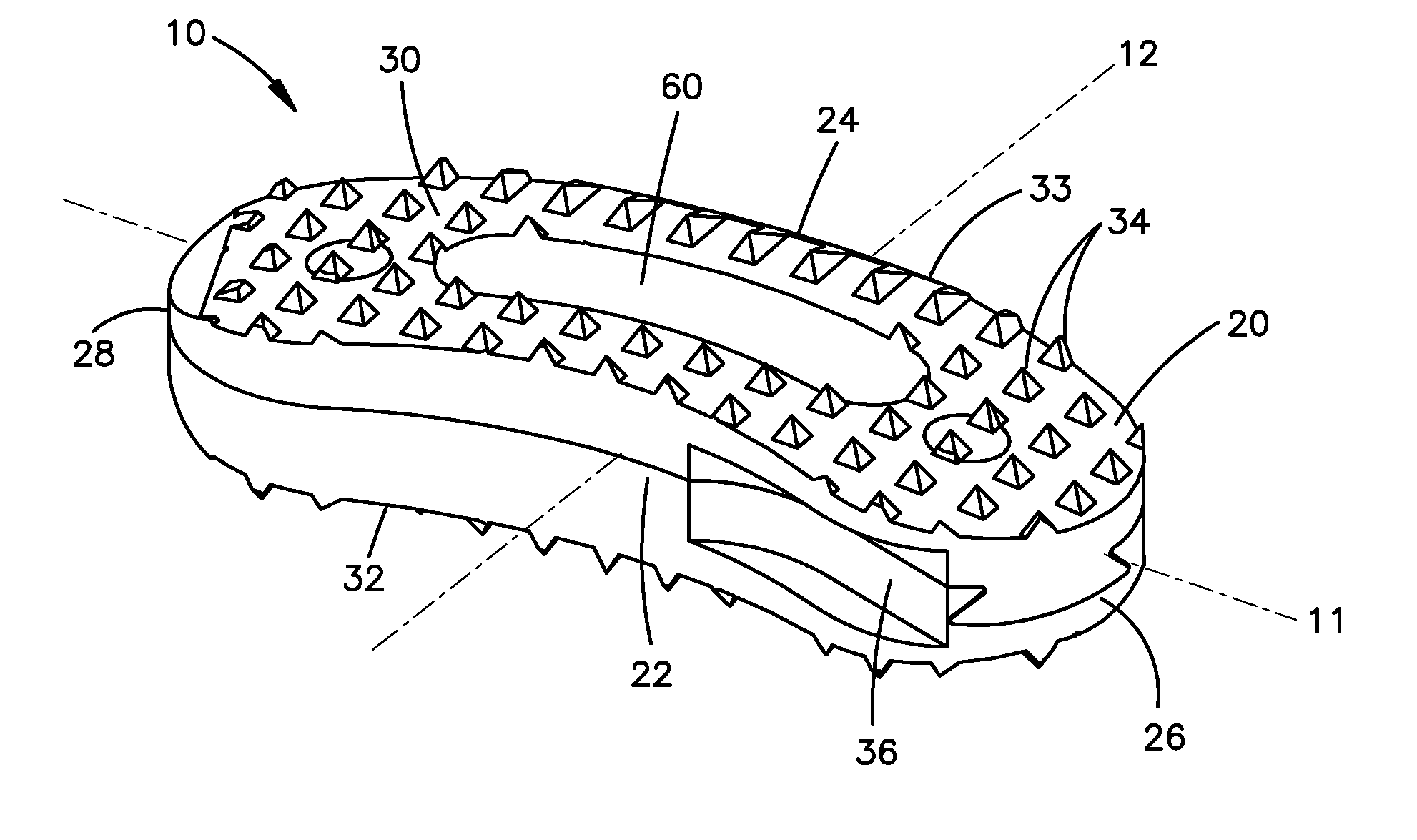
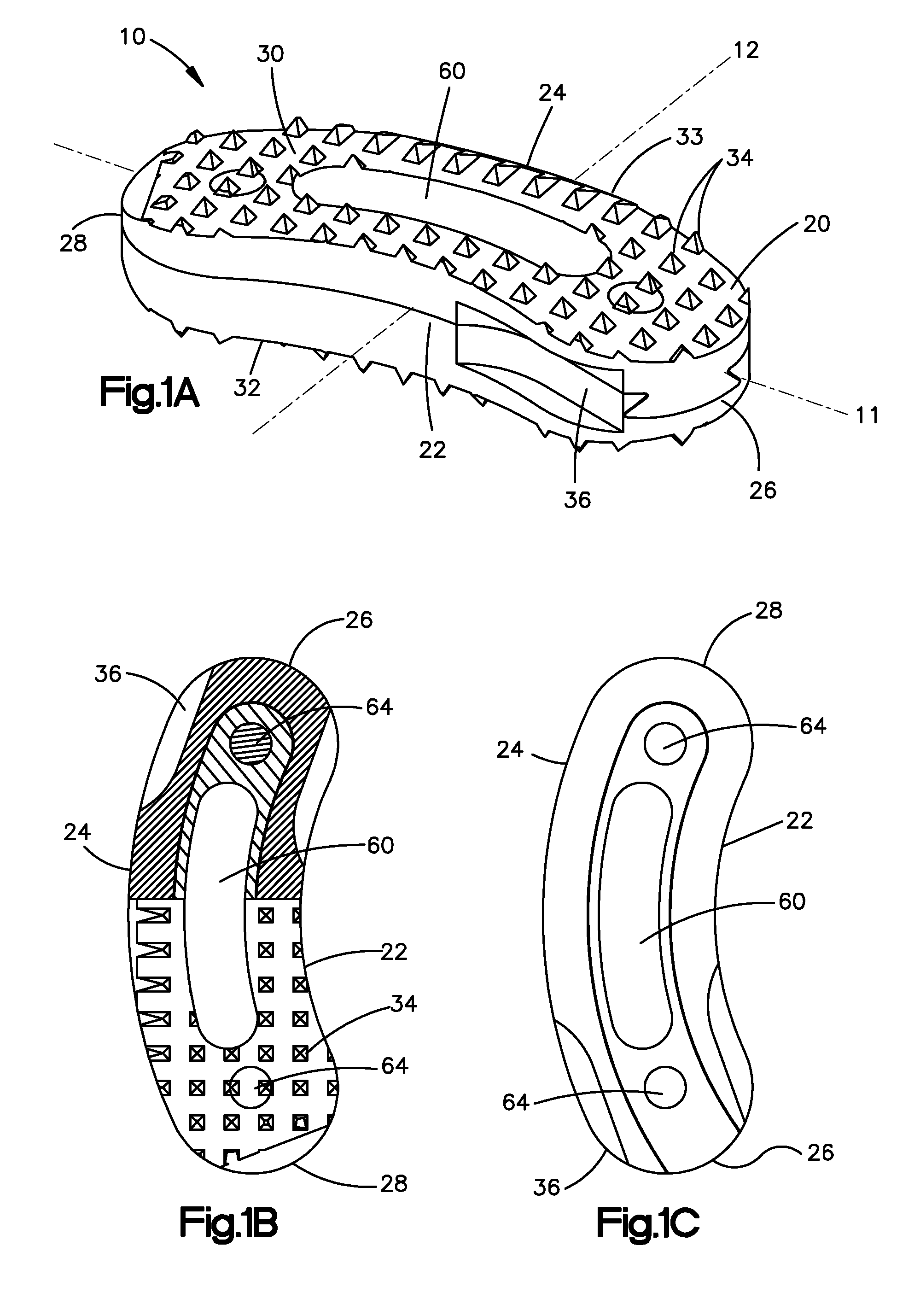
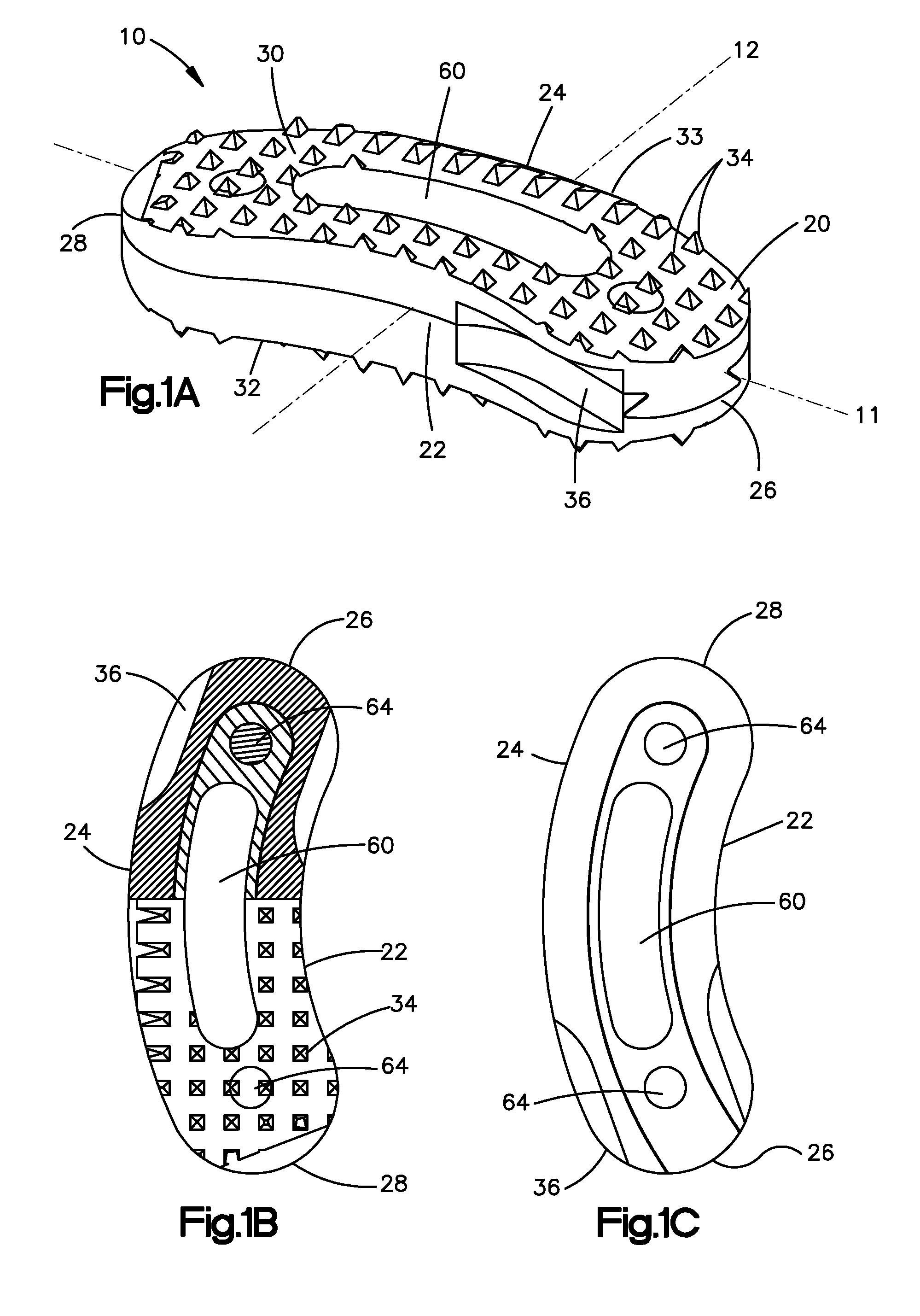
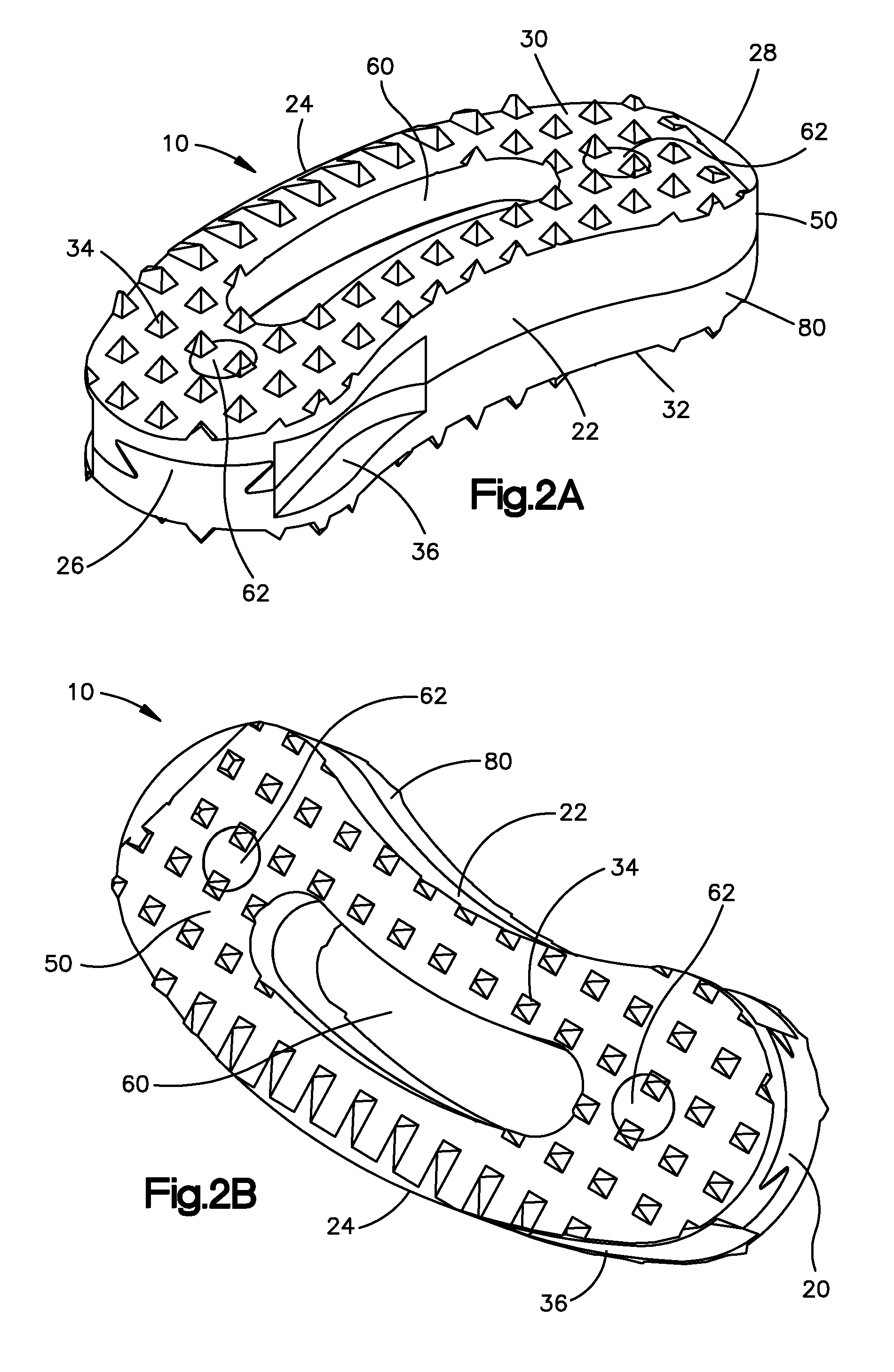
Bone grafts
InactiveUS20050004672A1Encourages bone ingrowthAvoid stress shieldingDiagnosticsBone implantBiomedical engineeringVertebra
Spinal spacers 20 are provided for fusion of a motion segment. The spacers include a load bearing member 21 having a wall 22 sized for engagement within a space between adjacent vertebrae to maintain the space and an effective amount of an osteogenic composition to stimulate osteoinduction. The osteogenic composition includes a substantially pure osteogenic factor in a pharmaceutically acceptable carrier. In one embodiment the load bearing member includes a bone graft impregnated in an osteogenic composition. In another embodiment, the osteogenic composition 30 is packed within a chamber 25 defined in the graft. Any suitable configuration of a bone graft is contemplated, including bone dowels, D-shaped spacers and cortical rings.
Owner:DANEK MEDICAL
Intervertebral prosthesis
An expandable intervertebral prosthesis includes a bone graft implant member dimensioned for insertion within an intervertebral space defined between adjacent vertebrae, thereafter adapted to vertically elevate and expand a plurality of barbs into the surrounding bone. The expandable intervertebral prosthesis has a tubular outer body portion having an axial bore with an enlarged proximal end and an exterior surface dimensioned to fit snugly within the space, and a barbed expansion cylinder slidably or rotatably mounted within the axial bore. The tubular outer body portion of the expandable intervertebral prosthesis has a plurality of longitudinal slots or holes in the wall thereof to allow the expansion and retraction of the expansion cylinder's barbs into or out of the surrounding bone. The barbs on the expansion cylinder may be elastically deformed from a normal, retracted configuration to a locking, splayed configuration wherein the outer ends of the barbs extend outwardly through the slots and exterior surface of tubular outer body to penetrate the surrounding bone as the expansion cylinder is moved. The expansion cylinder and, in one embodiment, the exterior surface of the tubular outer body portion, have a plurality of barbs disposed in circumferentially spaced relation about the body and positioned in various angles and positions respect to the axial bore. In another embodiment, the intervertebral prosthesis includes an elevating cylinder rotatably mounted within a frangible tubular outer body portion. The elevating cylinder has one or more detent positions that expand and vertically elevate the frangible tubular outer body portion of the intervertebral prosthesis body upon rotation thereof.
Owner:RHAUSLER
Guide pin for guiding instrumentation along a soft tissue tract to a point on the spine
InactiveUS20050137605A1Less immediate traumaConvenient amountInternal osteosythesisCannulasDistractionTreatments procedures
Disclosed are surgical tools, tool sets and methods for percutaneously accessing and preparing treatment sites within the spine for subsequent treatment procedures. The treatment site may be an inter-vertebral motion segments in the lumbar and sacral regions of the spine. The tool set may comprise introducer tools and bone dilators for accessing and tapping into a targeted site, such as, for example, the anterior surface of the S1 vertebral body. The tool set may also comprise cutters and extractors for preparing the treatment site for subsequent treatment procedures. The tool set may additionally comprise a bone graft inserter, an exchange system, and / or a temporary distraction tool for further preparing the treatment site for subsequent treatment procedures.
Owner:MIS IP HLDG LLC
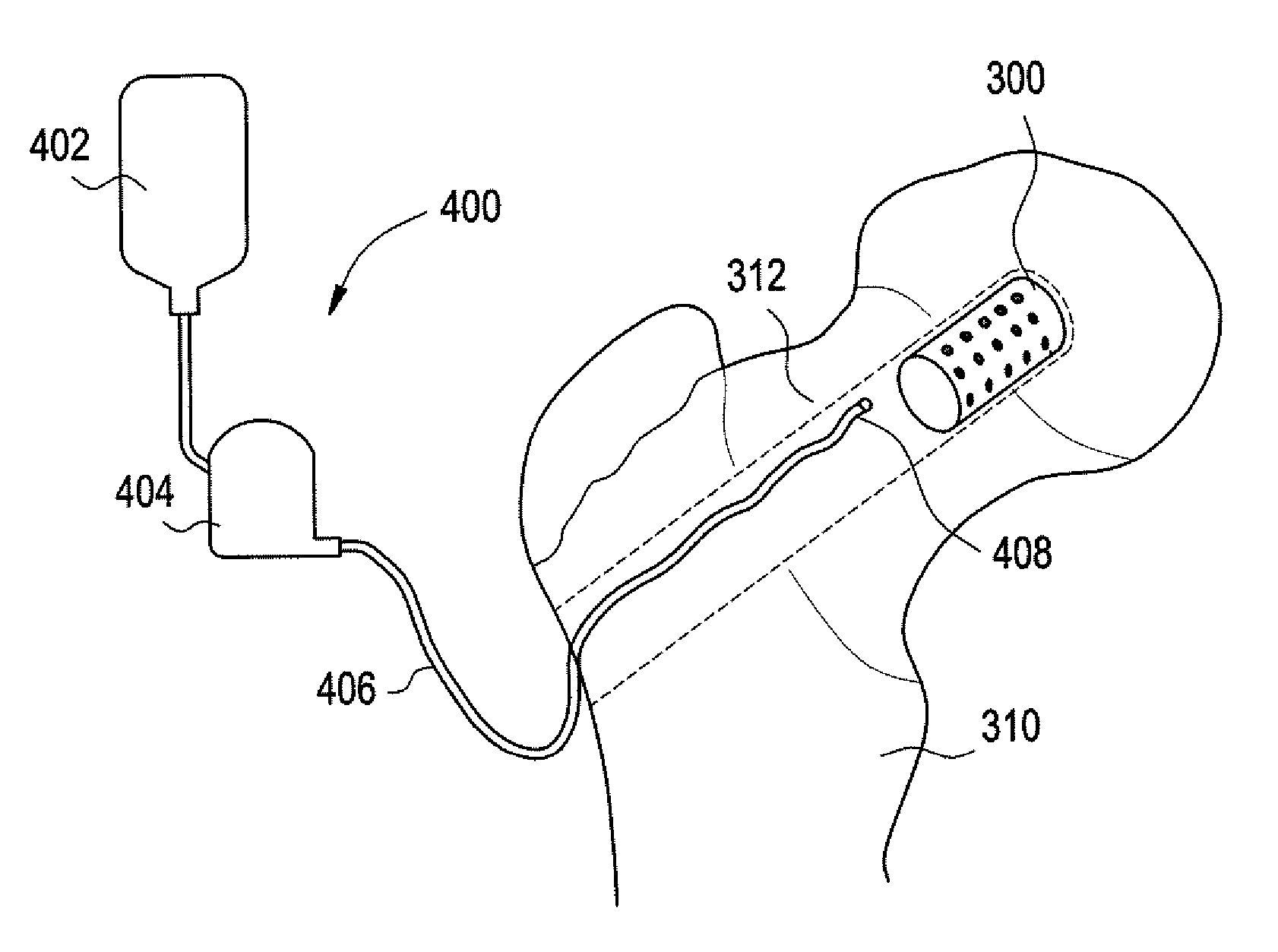
Balloon technologies for tissue repair
A medical device containing an inflatable balloon structure for use in minimally invasive surgery and minimally invasive diagnostic and therapeutic procedures are described herein. The device is delivered by a catheter and expanded using gases, liquids or liquids that solidify in situ. The inflatable balloon may be constructed from a wide variety of materials and may be reinforced by supporting structures, when necessary. The device may form an endoprosthesis in a patient. In the preferred embodiment, the device is used in spinal fusion. Optionally, the device may also be used in combination with bone graft materials and bioactive factors.
Owner:KUROS BIOSURGERY AG
Interbody spinal fusion device
InactiveUS20070276375A1Promote bone fusionEasy maintenanceInternal osteosythesisBone implantLamina terminalisAnterior surface
A pair of flat support plates in a spinal fusion implant device contact and rest against the softer, central cancellous bone portion of respective endplates of adjacent vertebrae. Each support plate has a front template that is orthogonal to the plate and is bent to communicate with the anterior surface of the hard cortical endplate the vertebrae. A wedge-shaped support strut in a central channel between the two plates is configured to vary the distance between the support plates such that the height of the device proximate the anterior end is greater than the height of the device at the posterior end to maintain the natural lordosis of the spine. Channels formed on either side of the support strut are filled with bone graft material and contact the endplates of the vertebrae through large openings in the flat support plates to facilitate fusion.
Owner:RAPP LAWRENCE G
Bioactive bone graft substitute
The invention relates to biocompatible bone graft materials for repairing bone defects and the application of such bone graft materials.
Owner:ORTHOVITA INC
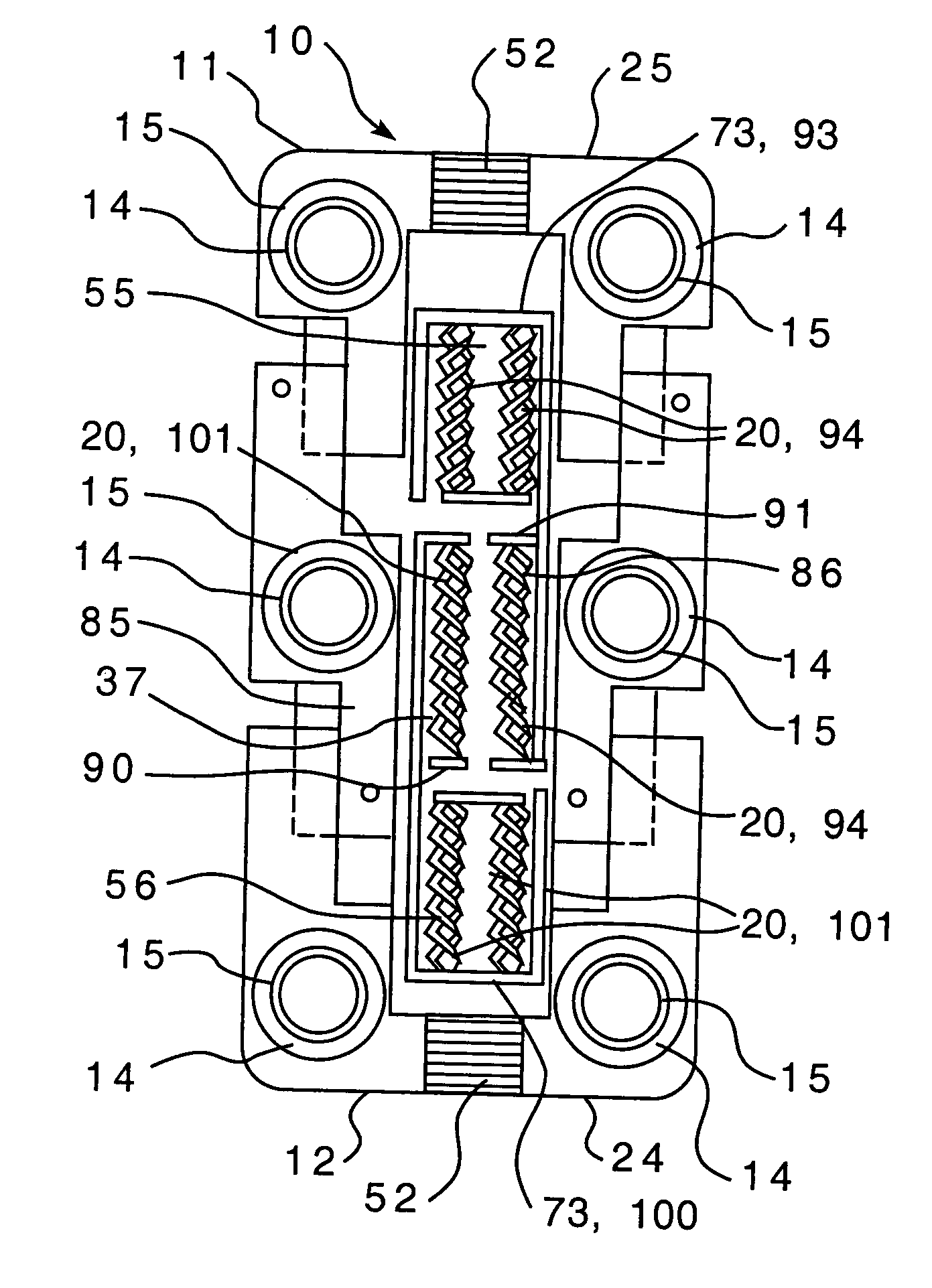
Biological delivery system with adaptable fusion cage interface
ActiveUS20160106551A1Control volumeIncreased traumaBone implantJoint implantsSurgical siteBiological materials
The present invention relates to an apparatus and method for near-simultaneous and integrated delivery of bone graft material during the placement of surgical cages or other medical implants in a patient's spine. The integrated fusion cage and graft delivery device according to various embodiments delivers and disperses biologic material through a fusion cage to a disc space and, without withdrawal from the surgical site, may selectably detach the fusion cage for deposit to the same disc space. The integrated fusion cage and graft delivery device is formed such that a hollow tube and plunger selectively and controllably place bone graft material and a fusion cage in or adjacent to the bone graft receiving area. In one embodiment, the integrated fusion cage is an expandable integrated fusion cage.
Owner:SPINAL SURGICAL STRATEGIES INC
Minimally Invasive Interbody Device Assembly
InactiveUS20080172128A1Restore heightEasy to placeInternal osteosythesisJoint implantsIntervertebral diskInstrumentation
A minimally invasive interbody device assembly that includes an interbody device that restores the disc space height between two vertebrae and an instrument detachably coupled to the interbody device for positioning the device in the disc space and delivering bone material to the disc space that is distributed on both sides of the interbody device. The device is inserted into the disc space using the instrument in a direction so that the wide dimension of the device is substantially parallel to the body of the vertebrae. The device is then rotated by the instrument so that the wide dimension of the device becomes perpendicular to the vertebral body so as to cause the disc space height to be restored. Bone graft material is then forced down the instrument so that the bone graft material is distributed on both sides of the device. The instrument is then detached from the device.
Owner:THOMPSON MIS
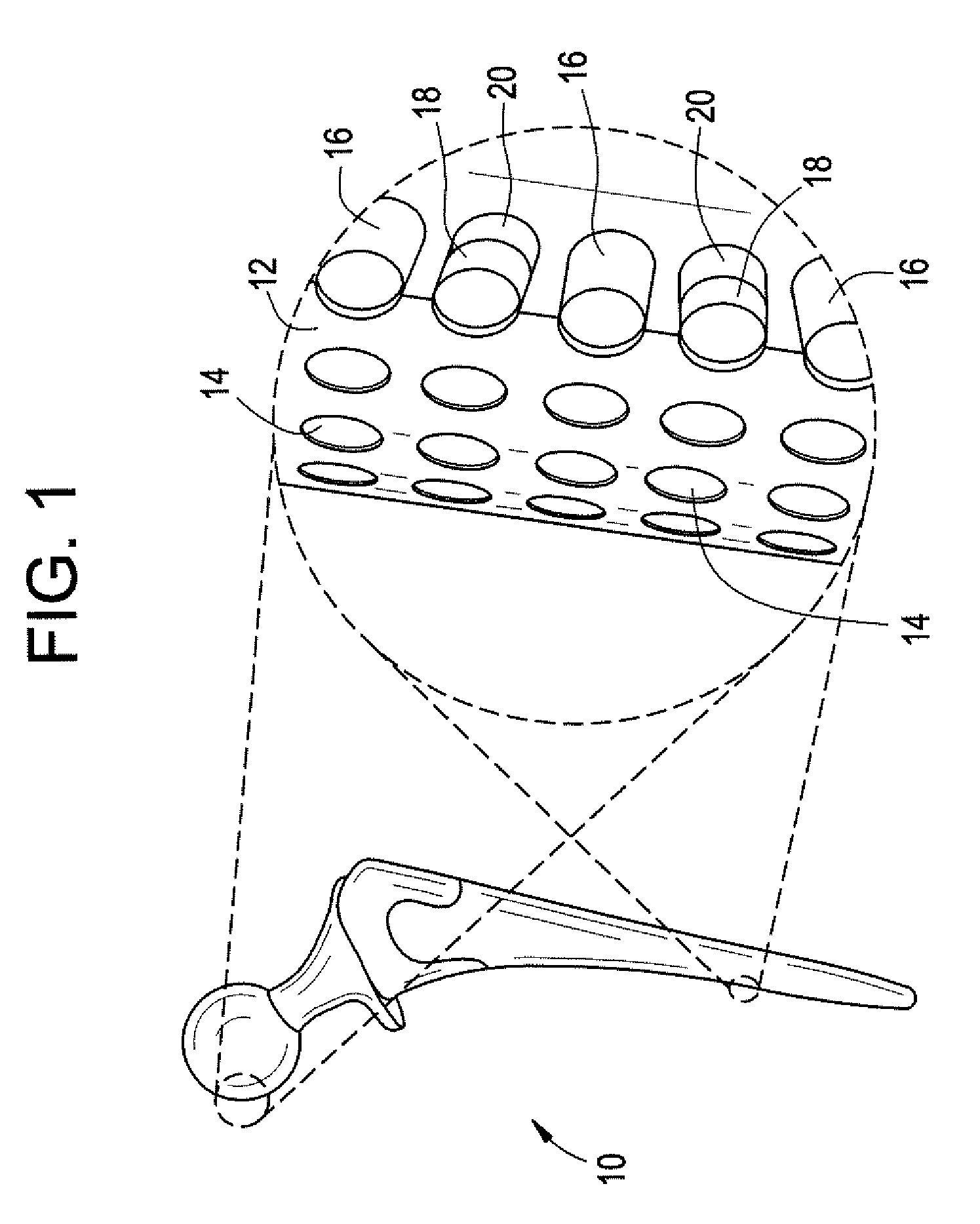
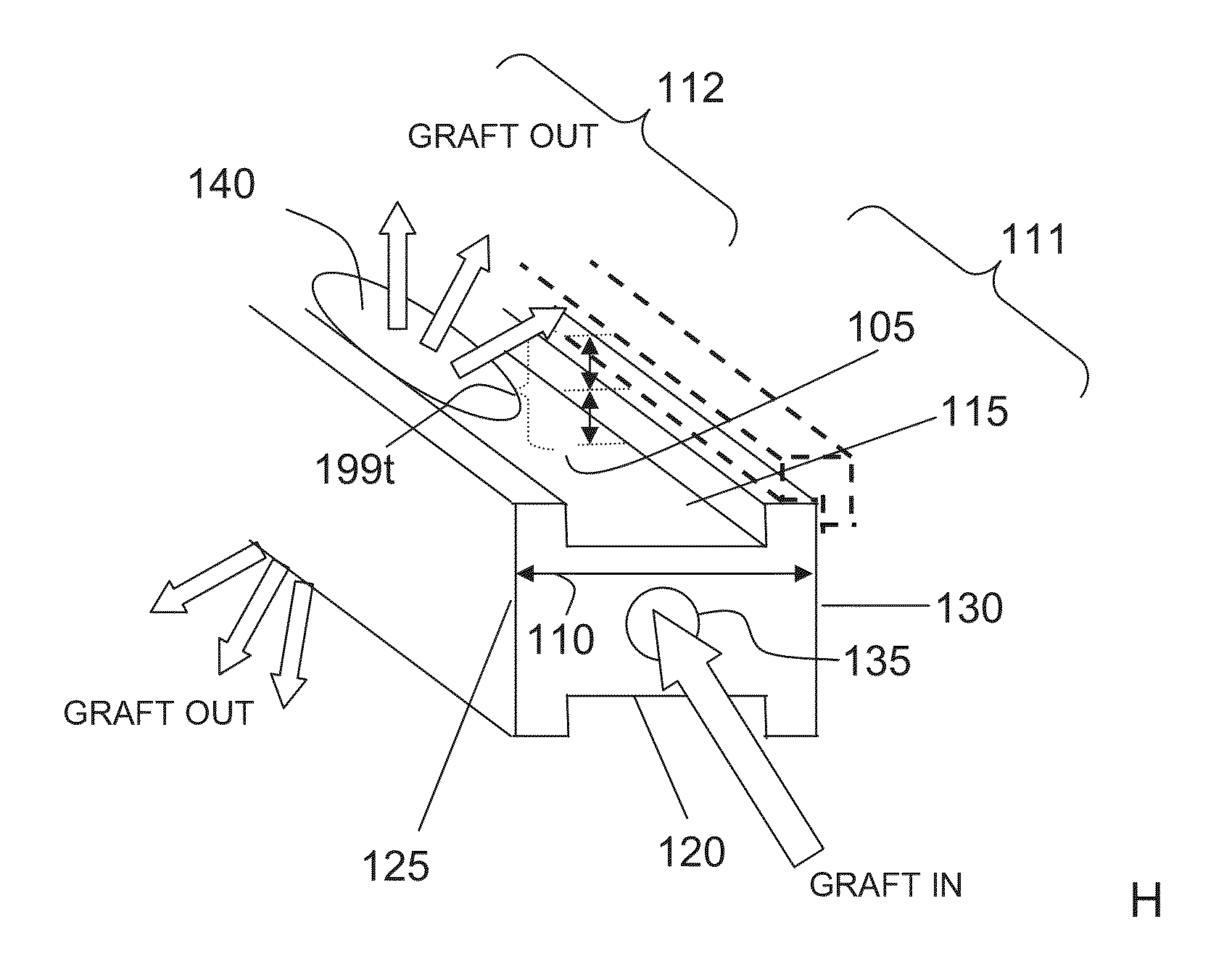
Bone graft distribution system
ActiveUS8663332B1Facilitate graft distributionBone implantSpinal implantsDistal portionDistribution system
A system for distributing bone graft material in an intervertebral disc space is provided having a central beam having a proximal portion having an end, a grafting portion having a top and a bottom, a distal portion having a end, a central beam axis, a graft distribution channel having an entry port at the end of the proximal portion, a top exit port at the top of the grafting portion, and a bottom exit port at the bottom of the grafting portion. These systems can also include a laterovertically-expanding frame operable for a reversible collapse from an expanded state into a collapsed state. The expanded state, for example, can be configured to have an open graft distribution window that at least substantially closes upon the reversible collapse.
Owner:INTEGRITY IMPLANTS INC
Selectively expanding spine cage, hydraulically controllable in three dimensions for enhanced spinal fusion
A selectively expanding spine cage has a minimized diameter in its unexpanded state that is smaller than the diameter of the neuroforamen through which it passes in the distracted spine. The cage conformably engages between the endplates of the adjacent vertebrae to effectively distract the anterior disc space, stabilize the motion segments and eliminate pathologic spine motion. The cage enhances spinal arthrodesis by creating a rigid spine segment. Expanding selectively, the cage height increases and holds the vertebrae with fixation forces greater than adjacent bone and soft tissue failure forces in natural lordosis. Stability is thus achieved immediately, enabling patient function by eliminating painful motion. The cage shape intends to rest proximate to the anterior column cortices securing the desired spread and fixation, allowing for bone graft in, around, and through the implant.
Owner:HOWMEDICA OSTEONICS CORP
Selectively expanding spine cage with enhanced bone graft infusion
A selectively expanding spine cage has a minimized cross section in its unexpanded state that is smaller than the diameter of the neuroforamen through which it passes in the distracted spine. The cage conformably engages between the endplates of the adjacent vertebrae to effectively distract the anterior disc space, stabilize the motion segments and eliminate pathologic spine motion. Expanding selectively (anteriorly, along the vertical axis of the spine) rather than uniformly, the cage height increases and holds the vertebrae with fixation forces greater than adjacent bone and soft tissue failure forces in natural lordosis. Stability is thus achieved immediately, enabling patient function by eliminating painful motion. The cage shape intends to rest proximate to the anterior column cortices securing the desired spread and fixation, allowing for bone graft in, around, and through the implant for arthrodesis whereas for arthroplasty it fixes to endpoints but cushions the spine naturally.
Owner:HOWMEDICA OSTEONICS CORP
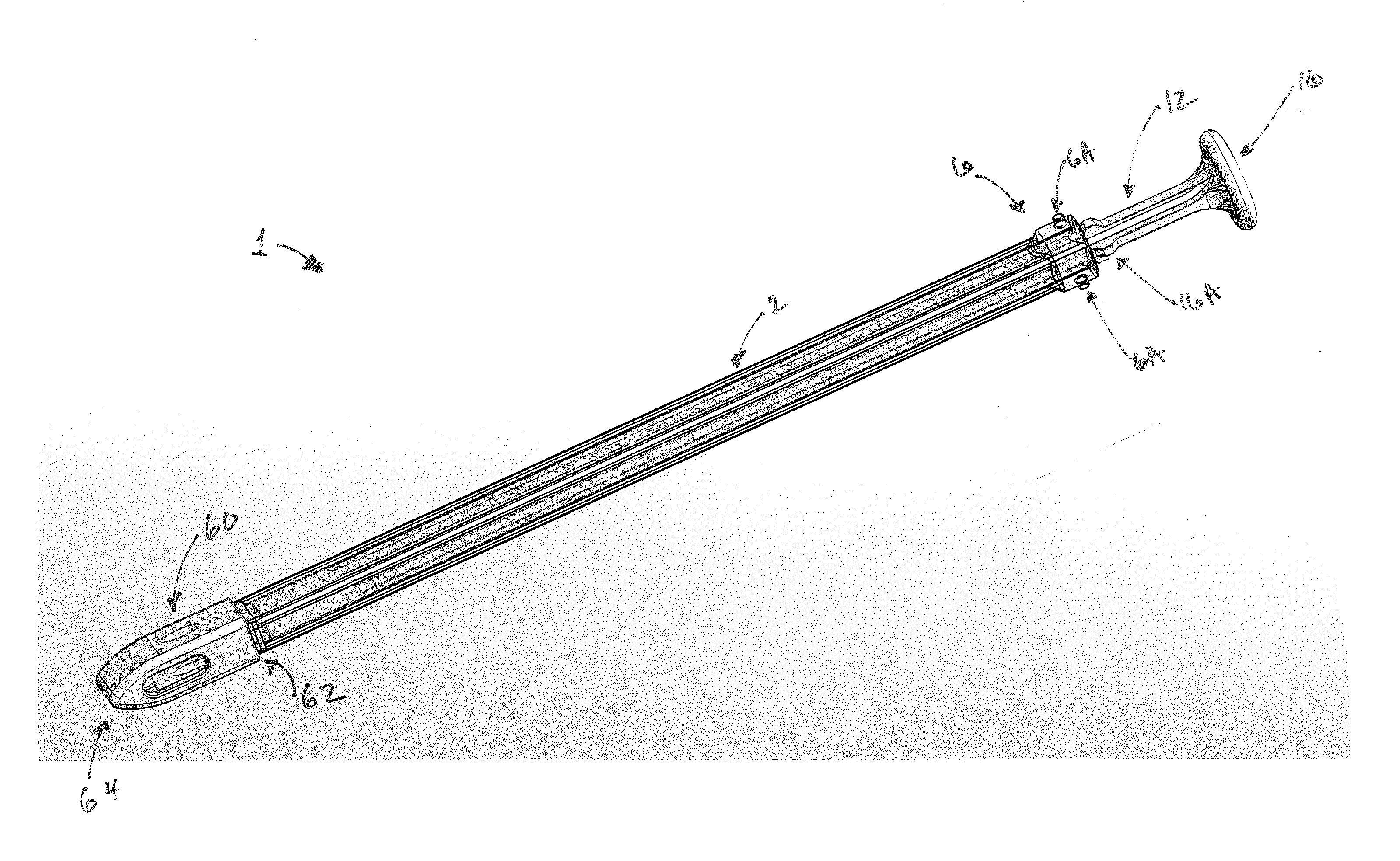
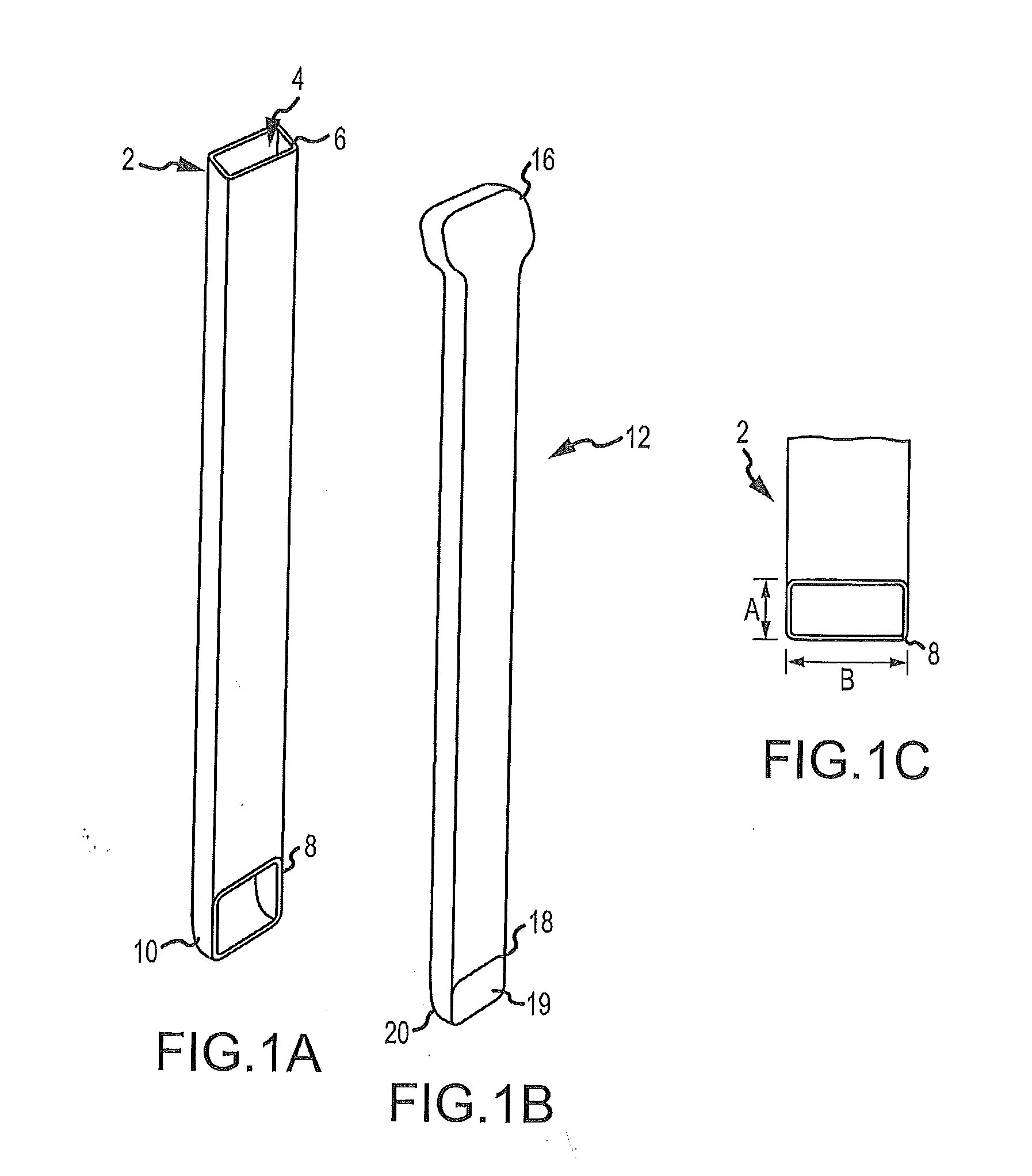
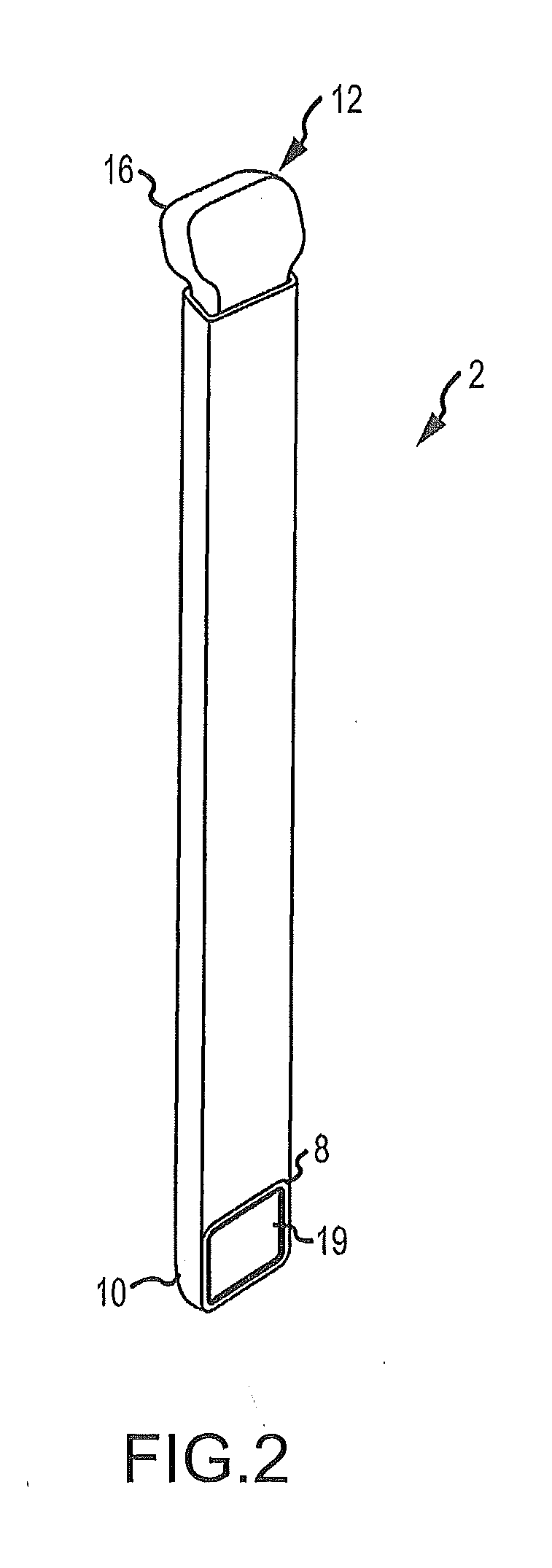
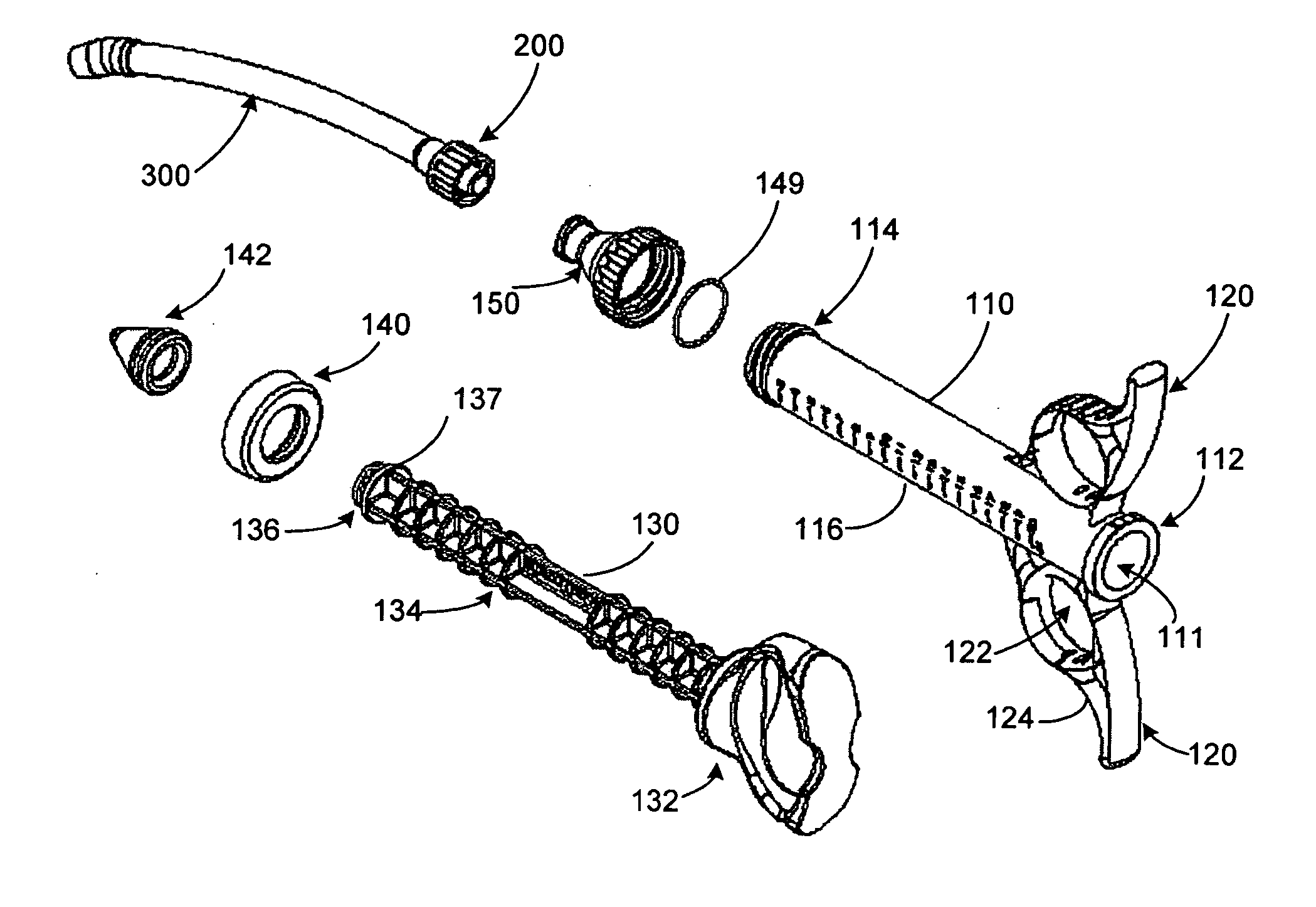
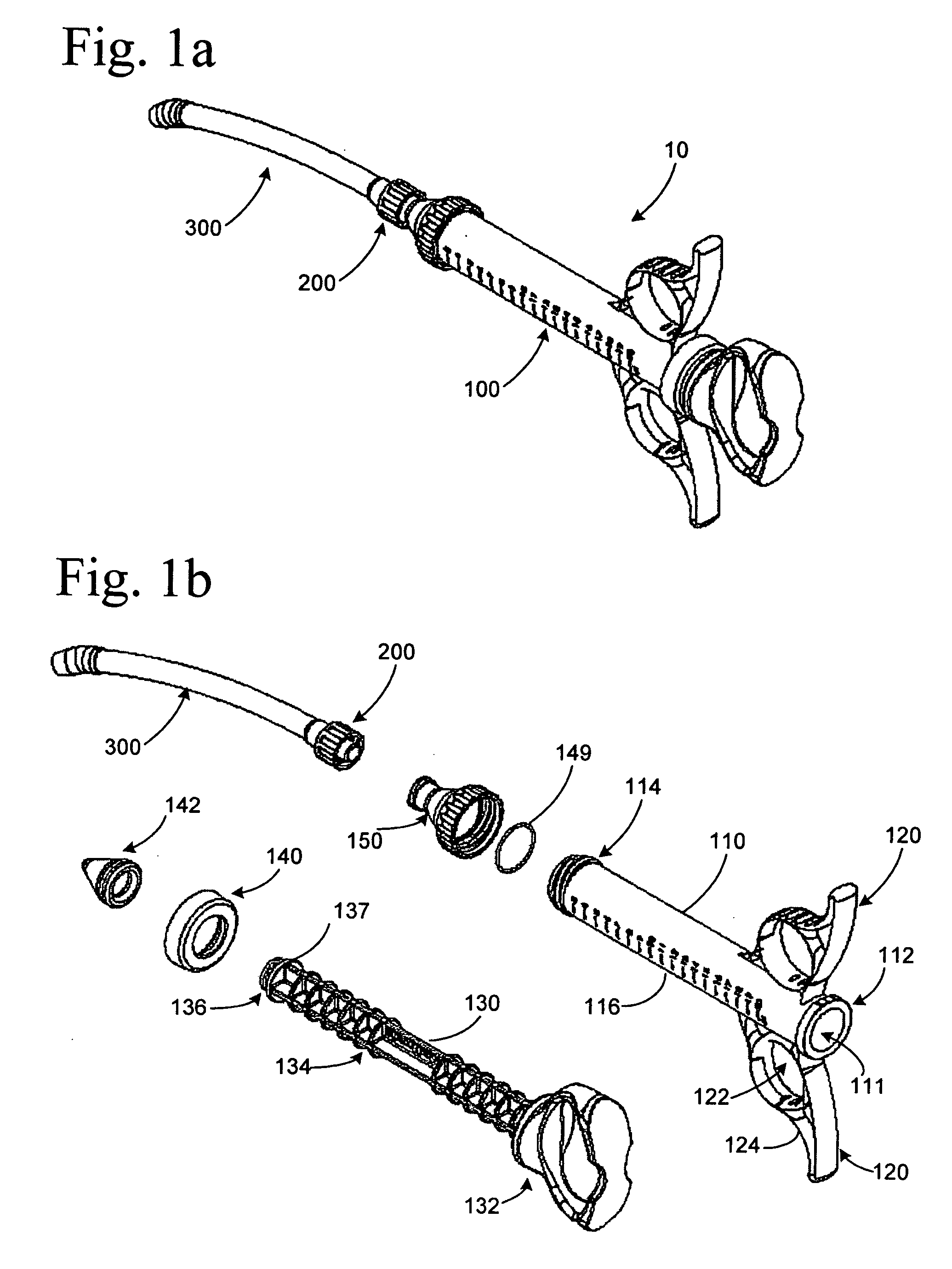
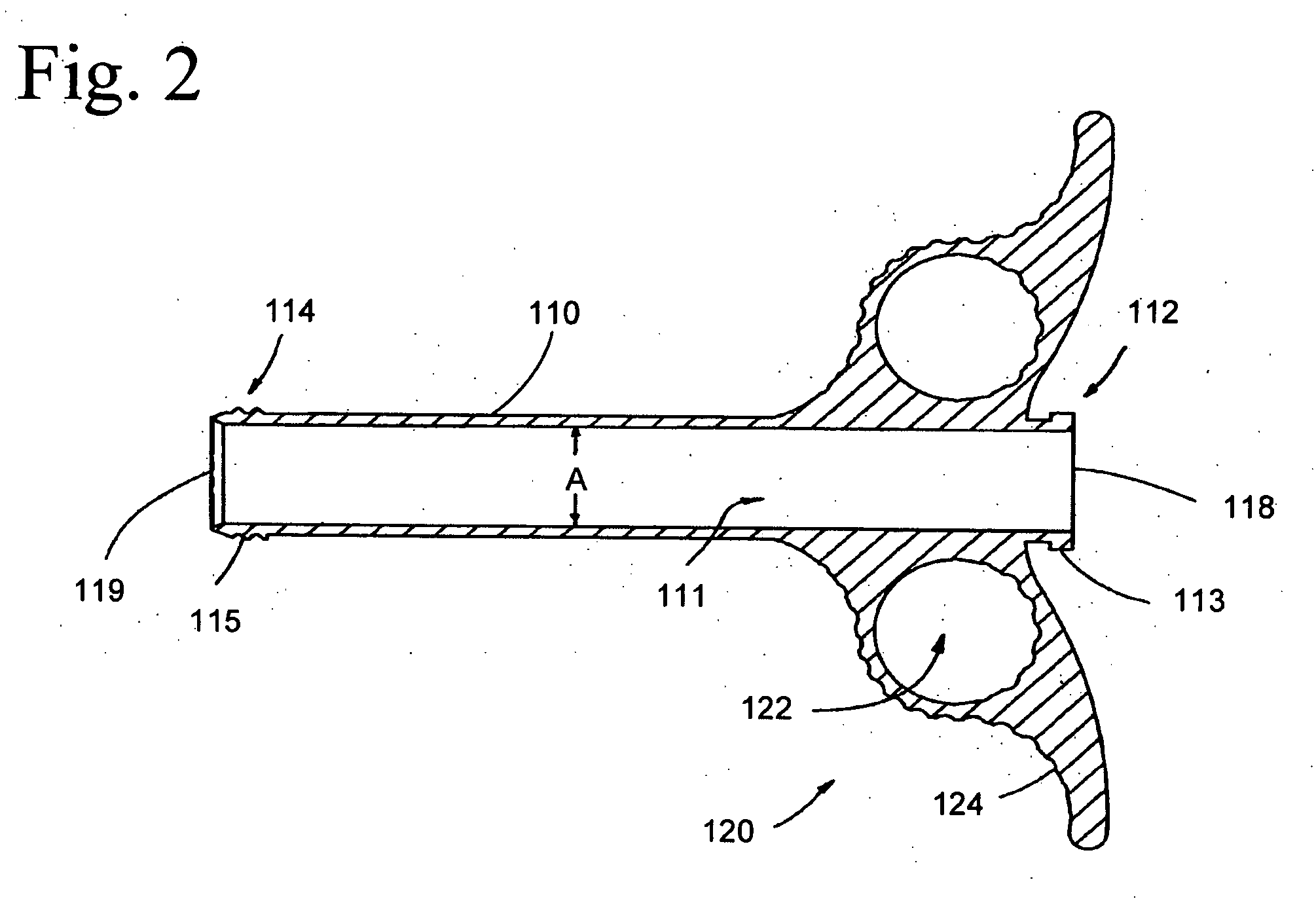
Graft syringe assembly
The invention relates, according to one embodiment, to a graft syringe assembly for delivering bone graft material is disclosed. The graft syringe assembly comprises a syringe subassembly including a syringe barrel having an inner chamber adapted for receiving bone graft material, a plunger adapted for expelling bone graft material from the inner chamber, the plunger slidably received within the inner chamber, and a syringe adapter coupled to the syringe barrel. The graft syringe assembly further comprises a connection subassembly coupled to the syringe adapter, and a delivery tube subassembly coupled to the connection subassembly, wherein the connection subassembly is configured to allow the delivery tube subassembly to rotate relative to the syringe subassembly.
Owner:WARSAW ORTHOPEDIC INC
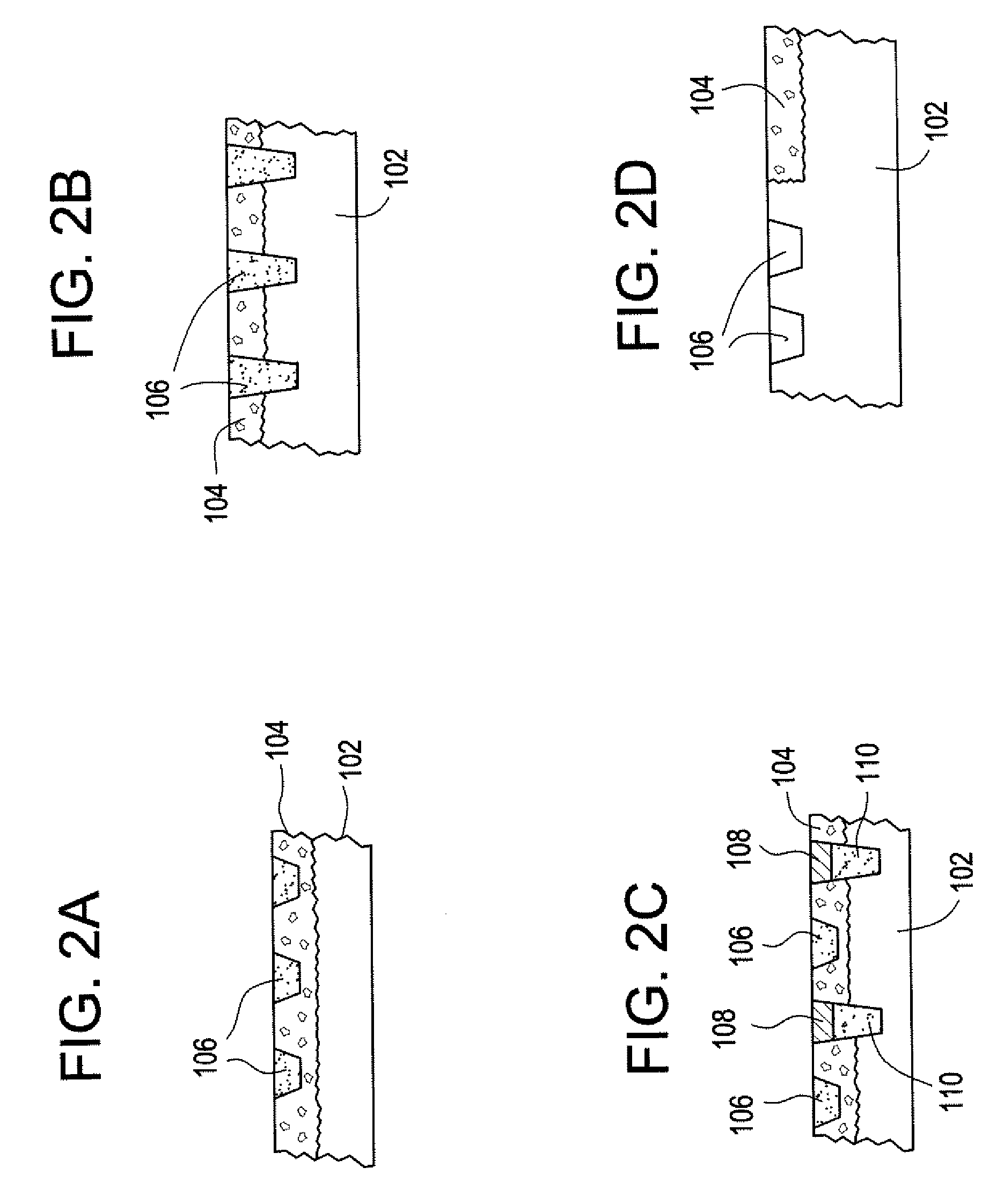
Synthetic bone substitute material
ActiveUS7250550B2Improve stabilityResorbed more quicklyBone implantUnknown materialsSulfateSynthetic bone
The invention includes a synthetic bone substitute material suitable for use as a replacement for cancellous bone in a bone graft composition, the material comprising a reticulated framework of interconnecting bioceramic struts defining an interconnecting interstitial void volume, and a solid non-porous composition substantially filling the interstitial void volume and in intimate contact with the reticulated framework, the pore-filling composition comprising calcium sulfate. Calcium triphosphate is a preferred bioceramic material for the reticulated framework.
Owner:WRIGHT MEDICAL TECH
Spinal fusion implants and devices and methods for deploying such implants
ActiveUS20140277481A1Add dimensionSpinal implantsOsteosynthesis devicesDistractionBone graft materials
Methods and apparatus are disclosed for distracting tissue. The devices and methods may include insertion of first and second elongated members into the space between two tissue layers, with an augmenting elongated member inserted therebetween to form a distraction device between the tissues to be distracted. The distraction device defines a generally annular configuration, with a locking member secured to one of the elongated members at a plurality of locations to maintain the distraction device in the generally annular configuration. The augmenting elongated member may be shorter than the first and second elongated members such that a window is defined between the proximal and distal ends of the augmenting elongated member when the distraction device and the first and second elongated members are in the generally annular configuration. Bone graft material or bone filler may be introduced into the interior of the distraction device through the window.
Owner:SPINAL ELEMENTS INC
Fusion cage with combined biological delivery system
ActiveUS20120136442A1Accurate maneuveringConvenient to accommodateBone implantSpinal implantsSurgical siteBiological materials
The present invention relates to an apparatus and method for near-simultaneous and integrated delivery of bone graft material during the placement of surgical cages or other medical implants in a patient's spine. The integrated fusion cage and graft delivery device according to various embodiments delivers and disperses biologic material through a fusion cage to a disc space and, without withdrawal from the surgical site, may selectably detach the fusion cage for deposit to the same disc space. The integrated fusion cage and graft delivery device is formed such that a hollow tube and plunger selectively and controllably place bone graft material and a fusion cage in or adjacent to the bone graft receiving area.
Owner:SPINAL SURGICAL STRATEGIES INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com