Naphthopyrone compounds, and preparation method and application thereof
A naphthopyrone and compound technology, which is applied in the field of naphthopyrone compounds and their preparation, can solve the problems of limited sources of coumarin substances, few varieties of clinical drugs, and poor targeting. Easy to handle, easy to obtain raw materials, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
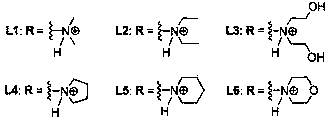
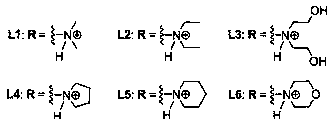
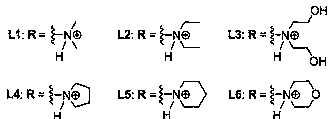
[0047] Example 1: Compound L1 - L6 preparation of
[0048] Table 1: Reagents used in the reactions and their abbreviations
[0049]
[0050] Proceed as follows:
[0051] 1) Pechmann condensation: first put 5.0g (31.24mmol) 1,5-naphthalenediol and 9.7mL (77.50mmol) ethyl acetoacetate into a 150mL three-necked flask, and stir for half an hour under the protection of nitrogen (use mechanical stirring ). Slowly add an appropriate volume of 80% concentrated sulfuric acid to the above-mentioned reaction device dropwise under ice bath conditions, remove the ice bath after the dropwise addition, stir at room temperature (25-27°C) for about 24 hours, and monitor the reaction process by TLC ( Ethyl acetate:petroleum ether=1:1, v / v), after confirming that there is no raw material, remove the reaction device, pour the reaction solution into 500mL ice water, let it stand overnight, and a yellow solid precipitates out. Filter, and then wash the filter cake to neutrality. After the ...
Embodiment 2
[0076] Example 2: The target naphthopyrone compound 12-17 of the present invention inhibits the growth of cancer cell A549 (lung adenocarcinoma)
[0077] Materials: A549 cells (human non-small cell lung cancer cells), six compounds L1 , L2 , L3, L4, L5, L6 , were dissolved in DMSO solution for use (10mM)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com