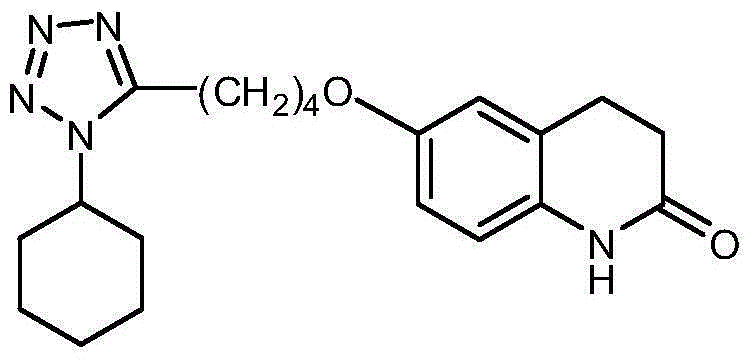
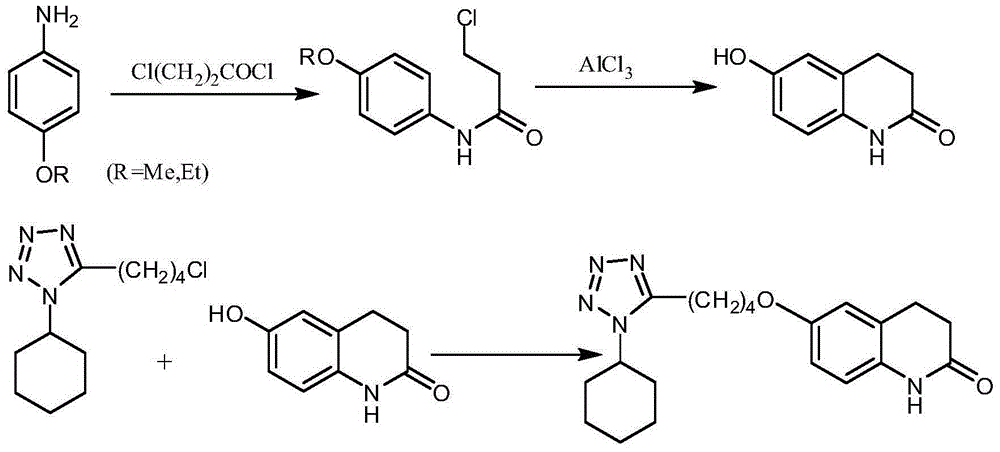
Method for synthesizing cilostazol
A technology of cilostazol and its synthetic method, which is applied in the field of organic chemical synthesis, can solve the problems of employee health hazards, poor atomic economy, and high raw material prices, and achieve the effects of reduced operational risk, less environmental pollution, and low raw material costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
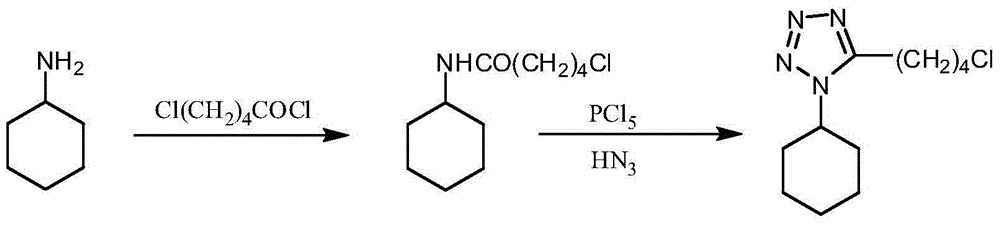
Embodiment 15
[0025] The preparation of embodiment 15-chloro-N-cyclohexylpentanamide
[0026] Put 49.6g (0.5mol) of cyclohexylamine into the reaction flask, add 200mL of tetrahydrofuran, 60mL of water, 75.9g of potassium carbonate (0.55mol), start stirring, cool to below 5°C, slowly add 77.5g of 5-chlorovaleryl chloride dropwise g (0.5mol), after dropping, react at room temperature for 2h, evaporate the solvent under reduced pressure, extract the residue with chloroform, separate layers, wash the organic phase with 0.1mol / L dilute hydrochloric acid and water successively until neutral, separate layers, water The layer was extracted twice with 50mL*2 chloroform, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the oil was added to 200mL of petroleum ether, stirred at room temperature, white crystals were gradually precipitated, filtered, and dried to obtain 5-chloro-N - Cyclohexylpentanamide 90.6g, yield 83.2%.
Embodiment 25
[0027] The preparation of embodiment 25-chloro-N-cyclohexylpentanamide
[0028] Put 49.6g (0.5mol) of cyclohexylamine into the reaction flask, add 200mL of methyl tetrahydrofuran, 60mL of water, 75.9g of potassium carbonate (0.55mol), start stirring, cool to below 5°C, and slowly add 5-chloropentyl Acyl chloride 77.5g (0.5mol), after dripping, react at room temperature for 2h, separate the layers, wash the organic layer with 0.1mol / L dilute hydrochloric acid and water until neutral, separate the layers, and extract the aqueous layer with 50mL*2 methyl tetrahydrofuran Twice, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure (methyl tetrahydrofuran was applied to the next batch), and the oil was added to 200 mL of petroleum ether, stirred at room temperature, and white crystals were gradually precipitated, filtered, and dried to obtain 5-chloro- N-cyclohexylpentanamide 96.6g, yield 88.7%.
Embodiment 35
[0029] The preparation of embodiment 35-(4-chloro-n-butyl)-1-cyclohexyl tetrazole
[0030] Add 95.8g (0.44mol) of 5-chloro-N-cyclohexylpentanamide to 400mL of toluene, start stirring, cool to below 5°C, slowly add 100.8g (0.48mol) of phosphorus pentachloride in batches, after the drop , kept at room temperature for 2 hours, added 60.7 g (0.53 mol) of azidotrimethylsilane, and reacted at room temperature for 15 hours. After the reaction was completed, 200 mL of water was added, and the layers were separated. The organic phase was washed with 100 mL of water, and then the toluene was evaporated under reduced pressure. Add 400mL of isopropanol, heat up to dissolve, keep warm for 1h, cool down, precipitate needle-like crystals, filter, and dry to obtain 97.4g of 5-(4-chloro-n-butyl)-1-cyclohexanetetrazole. The rate is 91.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com