High-stability simvastatin tablet and preparation method thereof
A high-stability, simvastatin technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problem of high requirements for excipients and equipment, high requirements for production equipment, and poor stability and other problems, to achieve the effect of suitable disintegration time, good fluidity and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
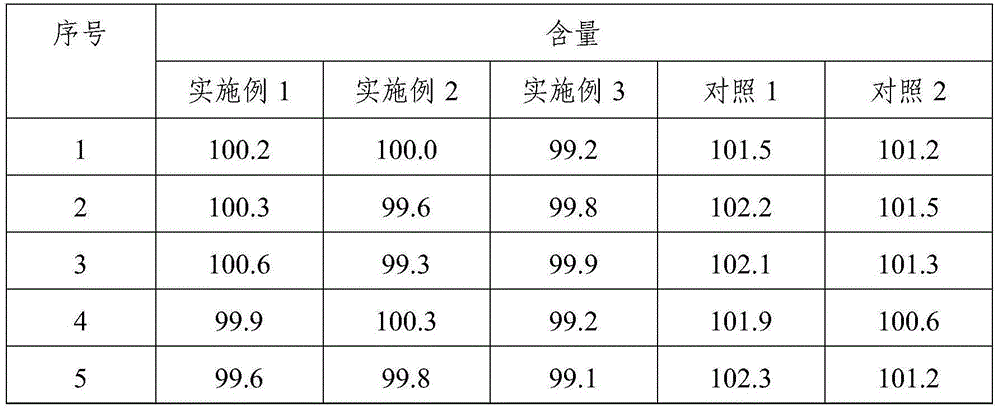
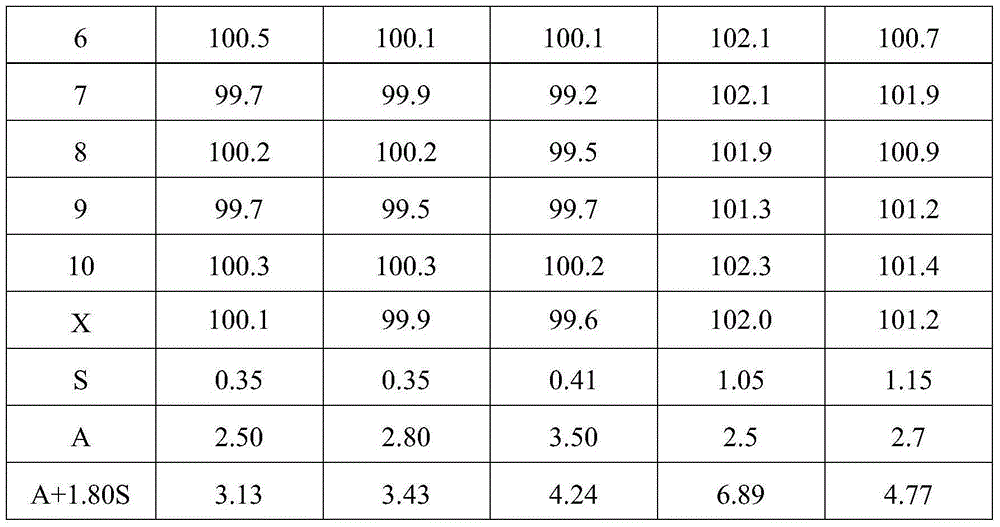
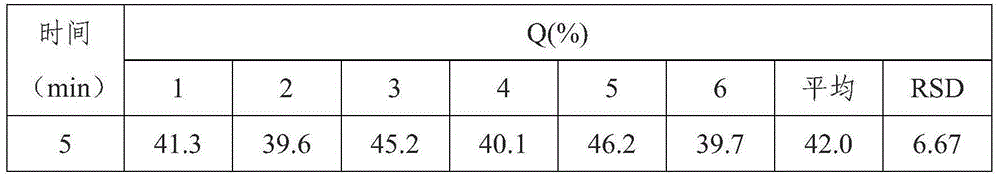
[0048] Simvastatin tablets include the following components: 10g of simvastatin, 15g of pregelatinized starch, 0.05g of butylated hydroxyanisole, 0.5g of povidone K, 50g of lactose, 40g of microcrystalline cellulose, 4g of hydroxypropyl cellulose, Hypromellose 1g, magnesium stearate 0.6g, micro silica gel 1.2g.
[0049] Above-mentioned simvastatin tablet is prepared by following method:
[0050] Weigh each component according to the formula, pass each component through an 80-mesh sieve, and set aside; bake the pregelatinized starch at 105°C for 2 hours, and set aside; dissolve povidone K30 in absolute ethanol to prepare mass Concentration is 12% dehydrated ethanol solution, standby; Hypromellose is dissolved in water B to make the aqueous solution with mass concentration of 2%, standby;
[0051] Mix simvastatin and pregelatinized starch evenly, dissolve butylated hydroxyanisole in absolute ethanol; use the above-mentioned povidone K30 absolute ethanol solution to make the abo...
Embodiment 2
[0056] Simvastatin tablets include the following components: 10g of simvastatin, 10g of pregelatinized starch, 0.04g of butylated hydroxyanisole, 300.3g of povidone K, 48g of lactose, 38g of microcrystalline cellulose, and 3.5g of hydroxypropyl cellulose , hypromellose 0.8g, magnesium stearate 0.4g, micro silica gel 0.8g.
[0057] Above-mentioned simvastatin tablet is prepared by following method:
[0058] Weigh each component according to the formula, pass each component through a 60-mesh sieve, and set aside; bake the pregelatinized starch at 100°C for 2 hours, and set aside; dissolve povidone K30 in absolute ethanol to make a mass 10% dehydrated ethanol solution for subsequent use; dissolving hypromellose in water ethyl alcohol to prepare an aqueous solution with a mass concentration of 1% for subsequent use;
[0059] Mix simvastatin and pregelatinized starch evenly, dissolve butyl hydroxyanisole in absolute ethanol; use the above-mentioned povidone K30 absolute ethanol so...
Embodiment 3
[0064] Simvastatin tablets include the following components: 20g of simvastatin, 15g of pregelatinized starch, 0.05g of butylated hydroxyanisole, 0.8g of povidone K, 52g of lactose, 42g of microcrystalline cellulose, and 4.5g of hydroxypropyl cellulose , hypromellose 1.5g, magnesium stearate 1.0g, micro silica gel 1.8g.
[0065] Above-mentioned simvastatin tablet is prepared by following method:
[0066] Weigh each component according to the formula, pass each component through a 100-mesh sieve, and set aside; bake the pregelatinized starch at 110°C for 3 hours, and set aside; dissolve povidone K30 in absolute ethanol to prepare mass Concentration is 15% absolute ethanol solution, for subsequent use; Hypromellose is dissolved in water B to make an aqueous solution with a mass concentration of 3%, for subsequent use;
[0067] Mix simvastatin and pregelatinized starch evenly, dissolve butylated hydroxyanisole in absolute ethanol; use the above-mentioned povidone K30 absolute et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com