Hypotensive drug urapidil hydrochloride composition water injection
A technology of urapidil and its composition, which is applied in the field of water injection of the antihypertensive drug urapidil composition, can solve the problems of increasing the risk of drug use for patients, the toxicity of patients, and affecting the quality of drugs, and achieves a level of fluidity suitable for clinical application. Improved, simple composition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of urapidil crystal
[0027] (1) Dissolving crude urapidil into a mixed solution of ethanol and carbon tetrachloride whose volume is 10 times the weight of uradil at 35°C, the volume ratio of ethanol and carbon tetrachloride is 4:2.5;
[0028] (2) Decolorize by adding activated carbon whose weight is 0.2 times the weight of urapidil, and filter to obtain urapidil solution;
[0029] (3) Warm up the uradil solution to 40°C, add propyl ether dropwise to the uradil solution under the condition of stirring, the volume of propyl ether is 8 times the weight of uradil, and the dropwise addition is completed at a uniform speed within 0.5h. The stirring rate is 30rmp;
[0030] (4) After the dropwise addition, cool down to -10°C at a rate of 15°C / hour, continue stirring at a stirring rate of 15rmp for 2h, let stand for 3h to precipitate crystals, filter, wash, and vacuum-dry to obtain urapidil crystals.
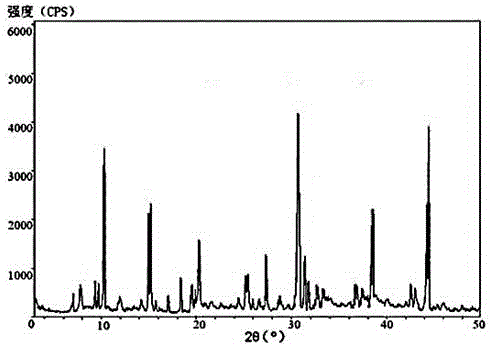
[0031] The X-ray powder diffraction pattern ...
Embodiment 2
[0032] Embodiment 2: the preparation of urapidil composition
[0033] The composition is: 1 part by weight of urapidil crystal prepared by the present invention, and 0.04 part by weight of calcium chloride.
[0034] The preparation method is:
[0035] Get the prescription amount of uradil and calcium chloride, add water for injection to dissolve, add 2% gac of uradil weight and stir at room temperature for 30 minutes, filter to remove carbon, add water for injection to the filtrate to 2000ml, pass through a 0.22 μm filter membrane, and divide Fill it into a 2ml ampoule, sterilize it by autoclaving at 121°C for 45 minutes, and inspect it with light to get urapidil water injection.
Embodiment 3
[0036] Embodiment 3: the preparation of urapidil composition
[0037] The composition is: 1 part by weight of urapidil crystal prepared by the present invention, and 0.05 part by weight of calcium chloride.
[0038] The preparation method is:
[0039] Get the prescription amount of uradil and calcium chloride, add water for injection to dissolve, add 2% gac of uradil weight and stir at room temperature for 30 minutes, filter to remove carbon, add water for injection to the filtrate to 2000ml, pass through a 0.22 μm filter membrane, and divide Fill it into a 2ml ampoule, sterilize it by autoclaving at 121°C for 45 minutes, and inspect it with light to get urapidil water injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com