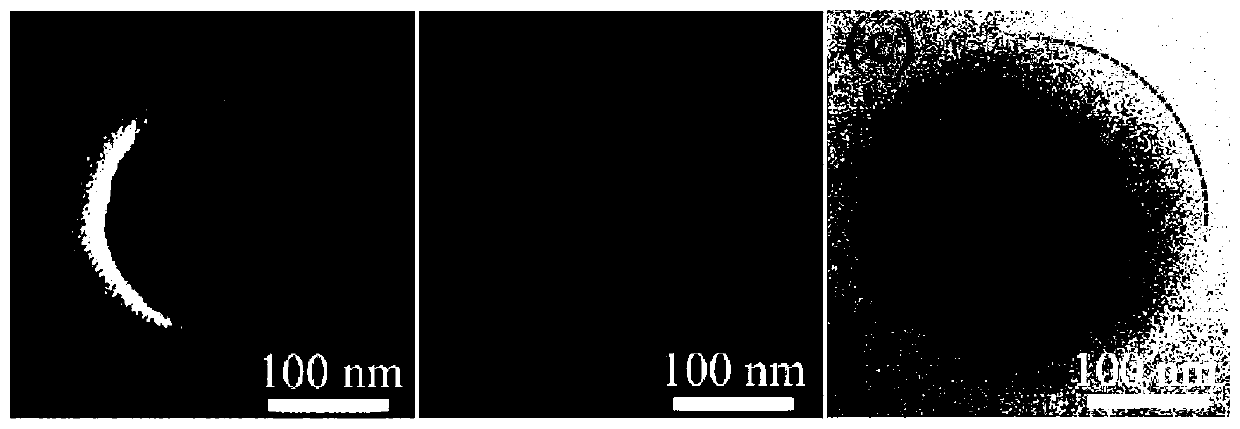
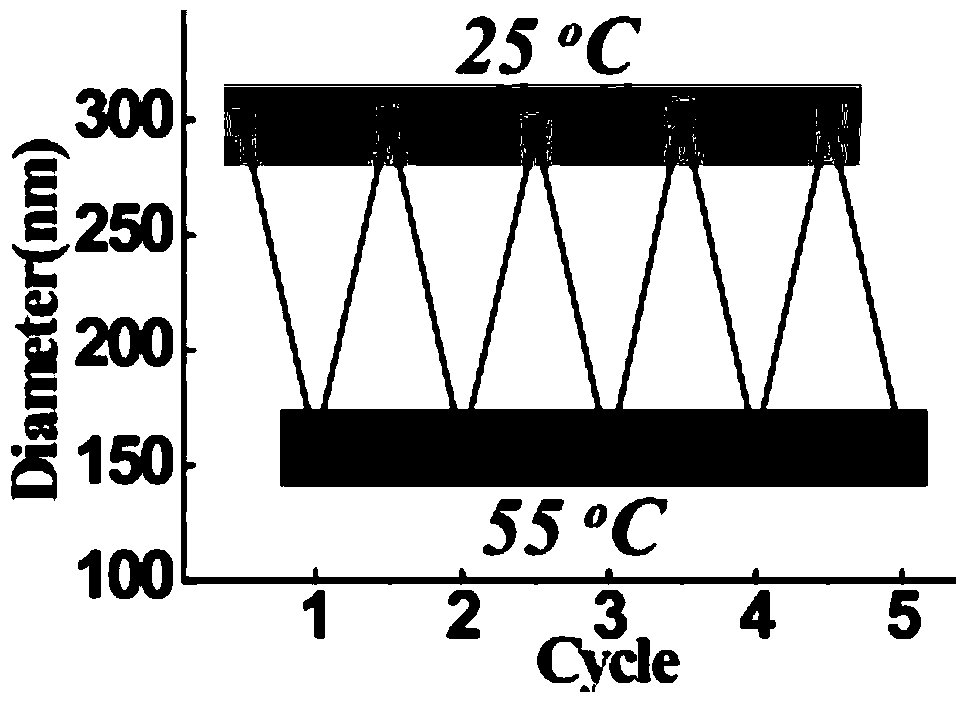
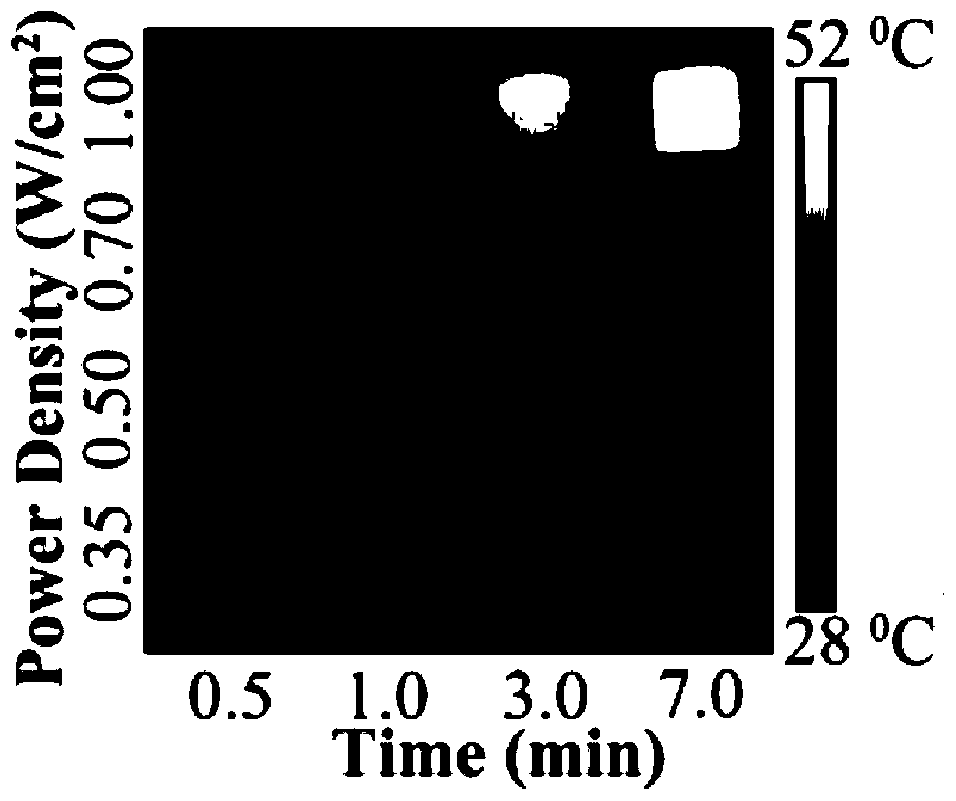
A near-infrared photothermal response anticancer drug nanocapsule and its preparation method
An anti-cancer drug, near-infrared light technology, applied in the directions of drug combination, microcapsule, capsule delivery, etc., can solve the problems of no longer having near-infrared photothermal effect, failure of photothermal nanomedicine capsules, and stability of gold nanorods. , to achieve long-term stable photothermal conversion efficiency, ultra-high photothermal conversion efficiency, and high drug loading effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] a.0.5mg Cu 1.75 S nanosheets were dispersed in 5.0mL chloroform, 8uL allyl mercaptan (Alm) was added and stirred slowly at 30°C for 30 minutes; after that, acetone was added to the solution, centrifuged (10000r / min, 10min), and then chloroform and acetone The excess Alm was washed away, and this purification process was repeated 3 times to obtain Cu 1.75 S@allylthiol nanoparticles were then dispersed into 1.0 mL of chloroform;
[0020] b. 1.0mL contains 0.5mg of Cu 1.75 The chloroform dispersion of S@allylthiol nanoparticles was mixed with 15 mL containing 300 mg N-isopropylacrylamide (NIPAM, monomer), 15 μg methacrylic acid (MAA, monomer) and 5 mg bisacrylamide (MBA, cross-linked). Joint agent) sodium dodecylsulfonate (SDS) solution (sodium dodecylsulfonate concentration 1.0mg / mL), under vigorous stirring and ultrasound, a brown emulsion was obtained; chloroform was evaporated at 58°C for 20 minutes; then Heat to 70°C, quickly add 1.0mL (10mg / mL) potassium persulfat...
Embodiment 2
[0024] a.0.5mg Cu 1.75 S nanosheets were dispersed in 5.0mL chloroform, 8uL allyl mercaptan (Alm) was added and stirred slowly at 30°C for 30 minutes; after that, acetone was added to the solution, centrifuged (10000r / min, 10min), and then chloroform and acetone The excess Alm was washed away, and this purification process was repeated 3 times to obtain Cu 1.75 S@allylthiol nanoparticles were then dispersed into 1.0 mL of chloroform;
[0025] b. 1.0mL contains 0.5mg of Cu 1.75 The chloroform dispersion of S@allylthiol nanoparticles was mixed with 15 mL containing 300 mg N-isopropylacrylamide (NIPAM, monomer), 15 μg methacrylic acid (MAA, monomer) and 10 mg bisacrylamide (MBA, monomer). Sodium dodecylsulfonate (SDS) solution (1.0mg / mL), under vigorous stirring and ultrasonication, a brown emulsion was obtained; chloroform was evaporated at 58°C for 20 minutes; then heated to 70°C, and 1.0 mL (10mg / mL) potassium persulfate (KPS, initiator) solution, maintained at 70°C for 5 h...
Embodiment 3
[0028] a.0.5mg Cu 1.75S nanosheets were dispersed in 5.0mL chloroform, 8uL allyl mercaptan (Alm) was added and stirred slowly at 30°C for 30 minutes; after that, acetone was added to the solution, centrifuged (10000r / min, 10min), and then chloroform and acetone The excess Alm was washed away, and this purification process was repeated 3 times to obtain Cu 1.75 S@allylthiol nanoparticles were then dispersed into 1.0 mL of chloroform;
[0029] b. 1.0mL contains 0.5mg of Cu 1.75 The chloroform dispersion of S@allylthiol nanoparticles was mixed with 15 mL containing 300 mg N-isopropylacrylamide (NIPAM, monomer), 15 μg methacrylic acid (MAA, monomer) and 15 mg bisacrylamide (MBA, monomer). Sodium dodecylsulfonate (SDS) solution (1.0mg / mL), under vigorous stirring and ultrasonication, a brown emulsion was obtained; chloroform was evaporated at 58°C for 20 minutes; then heated to 70°C, and 1.0 mL (10mg / mL) potassium persulfate (KPS, initiator) solution, maintained at 70°C for 5 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com