Method for synthesizing carbamic ester through alkyl boronic acid, amine and carbon dioxide
A carbamate, hydrocarbyl boronic acid technology, applied in the preparation of carbamate derivatives, organic chemistry methods, chemical instruments and methods, etc., can solve problems such as life-threatening safety and environmental pollution, and achieve safe, simple and adaptable reaction operations. The effect of good and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
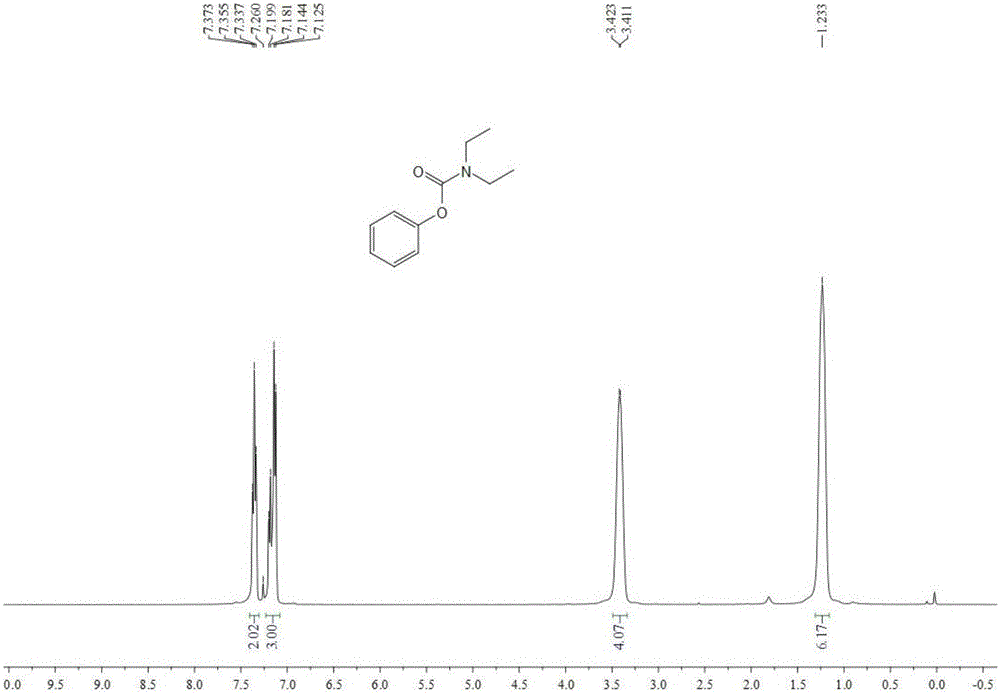
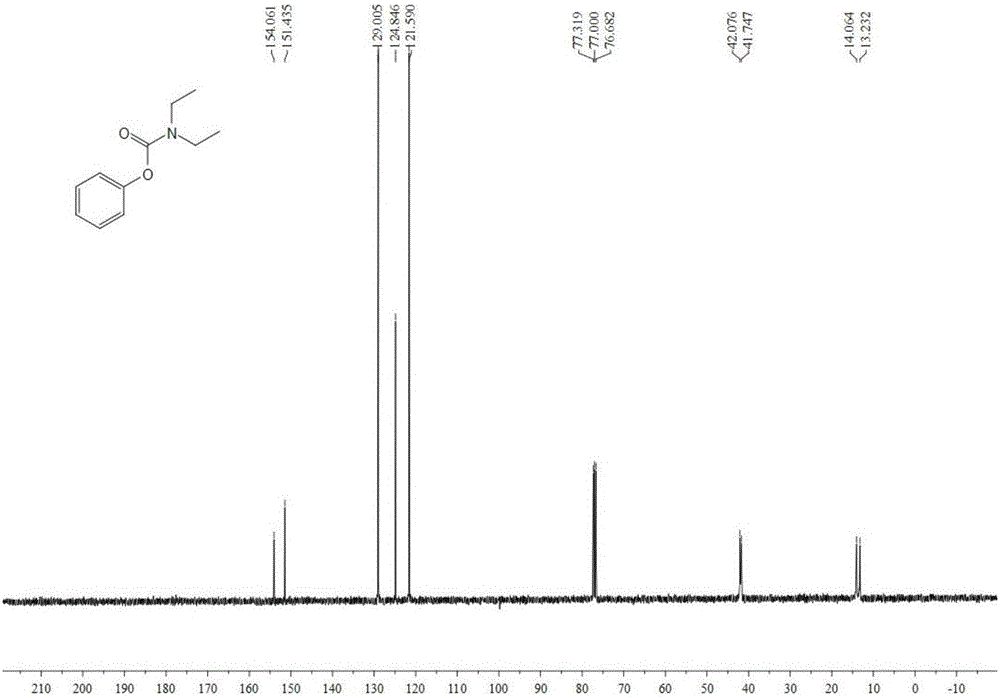
Image
Examples
Embodiment 1
[0036] Add 0.5 mmol phenylboronic acid, 0.2 mmol cuprous oxide, 3 ml dichloromethane, 2.5 mmol diethylamine, 1.5 mmol boron trifluoride diethyl ether, 1.5 mmol pyridine to the autoclave, first fill 0.4 Oxygen in MPa, and then filled with carbon dioxide to make the total pressure reach 4MPa. After stirring and reacting at 80°C for 24 hours, stop heating and stirring, cool to room temperature, and slowly vent unreacted CO 2 . The reaction solution was extracted with ethyl acetate, filtered, and the solvent was removed by rotary evaporation under reduced pressure to obtain a crude product, which was then separated and purified by column chromatography to obtain the target product. The eluent of the column chromatography used was petroleum ether with a volume ratio of 20:1 : ethyl acetate mixed solvent, productive rate 61%.
Embodiment 2
[0038] Add 0.5 mmol phenylboronic acid, 0.2 mmol cuprous oxide, 3 ml dichloromethane, 2.5 mmol diethylamine, 1 mmol boron trifluoride diethyl ether, 1.5 mmol pyridine to the autoclave, and first fill 0.4 Oxygen in MPa, and then filled with carbon dioxide to make the total pressure reach 4MPa. After stirring and reacting at 80°C for 24 hours, stop heating and stirring, cool to room temperature, and slowly vent unreacted CO 2. The reaction solution was extracted with ethyl acetate, filtered, and the solvent was removed by rotary evaporation under reduced pressure to obtain a crude product, which was then separated and purified by column chromatography to obtain the target product. The eluent of the column chromatography used was petroleum ether with a volume ratio of 20:1 : ethyl acetate mixed solvent, productive rate 39%.
Embodiment 3
[0040] Add 0.5 mmol phenylboronic acid, 0.05 mmol cuprous oxide, 3 ml dichloromethane, 2.5 mmol diethylamine, 1.5 mmol boron trifluoride diethyl ether, 1.5 mmol pyridine into the autoclave, and first fill 0.2 Oxygen in MPa, and then filled with carbon dioxide to make the total pressure reach 4MPa. After stirring and reacting at 80°C for 24 hours, stop heating and stirring, cool to room temperature, and slowly vent unreacted CO 2 . The reaction solution was extracted with ethyl acetate, filtered, and the solvent was removed by rotary evaporation under reduced pressure to obtain a crude product, which was then separated and purified by column chromatography to obtain the target product. The eluent of the column chromatography used was petroleum ether with a volume ratio of 20:1 : ethyl acetate mixed solvent, productive rate 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com