Liquid chromatographic method for separating and determining multiple impurities in tolvaptan
A technology of tolvaptan and high-performance liquid chromatography, applied in the field of drug analysis, to achieve the effects of improved safety, strong specificity, and accurate and reliable determination results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 Detection condition of the present invention
[0061] High performance liquid chromatography: LC-20AT, SPD-20A
[0062] Column: Symmetry shield TM RP18 (4.6×150mm, 5μm);
[0063] Mobile phase: acetonitrile-water (containing 0.2% phosphoric acid) (45:55);
[0064] Flow rate: 1.0mL / min;
[0065] Detection wavelength: 254nm;
[0066] Column temperature: 25°C;
[0067] Injection volume: 10 μL.
[0068] Detection steps:
[0069] Take an appropriate amount of the reference substances of impurities II, III, IV, V and VI, dissolve them in methanol, and prepare a reference substance solution containing about 6.0 μg per 1 mL
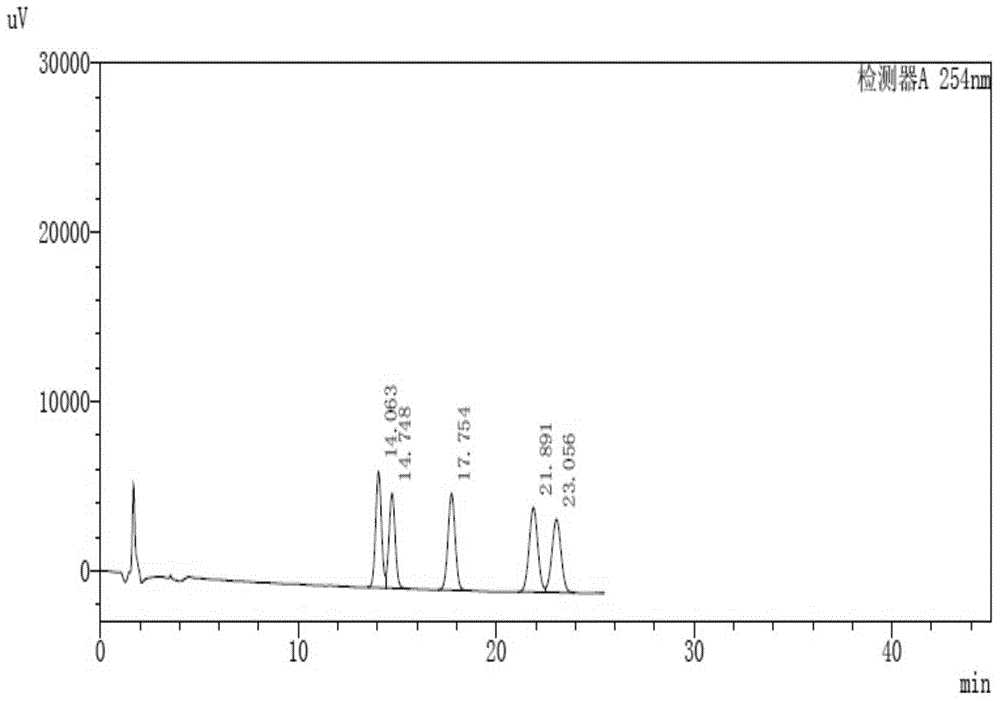
[0070] Determination method: Take 10 μL of the above solution and inject it into the liquid chromatograph, record the chromatogram, the result is as follows: figure 1 shown. The chromatographic peak separation degree of impurity Ⅱ and impurity Ⅲ in the spectrum is 1.518; the chromatographic peak separation degree of impurity Ⅴ and imp...
Embodiment 2
[0119] Embodiment 2 Detection of tolvaptan and its impurities
[0120] Detection conditions:
[0121] High performance liquid chromatography: LC-20AT, SPD-20A
[0122] Column: Symmetry shield TM RP18 (4.6×150mm, 5μm);
[0123] Mobile phase: acetonitrile-water (containing 0.2% phosphoric acid) (45:55);
[0124] Flow rate: 1.0mL / min;
[0125] Detection wavelength: 254nm;
[0126] Column temperature: 25°C;
[0128] Injection volume: 10 μL.
[0129] a, get tolvaptan I (crude product) 10mg, put in 10mL measuring bottle, add solvent to dissolve and dilute to scale, as need testing solution;
[0130] b. Take an appropriate amount of impurity II, III, IV, V and VI reference substances, dissolve and dilute them with solvents respectively to make reference substance solutions with a concentration of about 1.0 μg / mL;
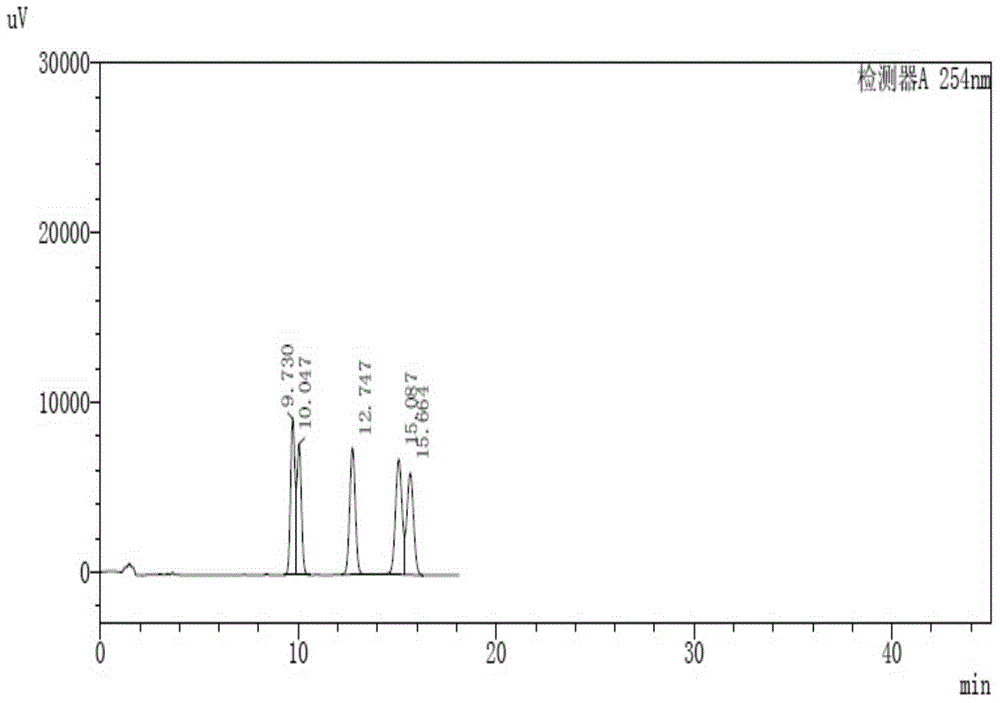
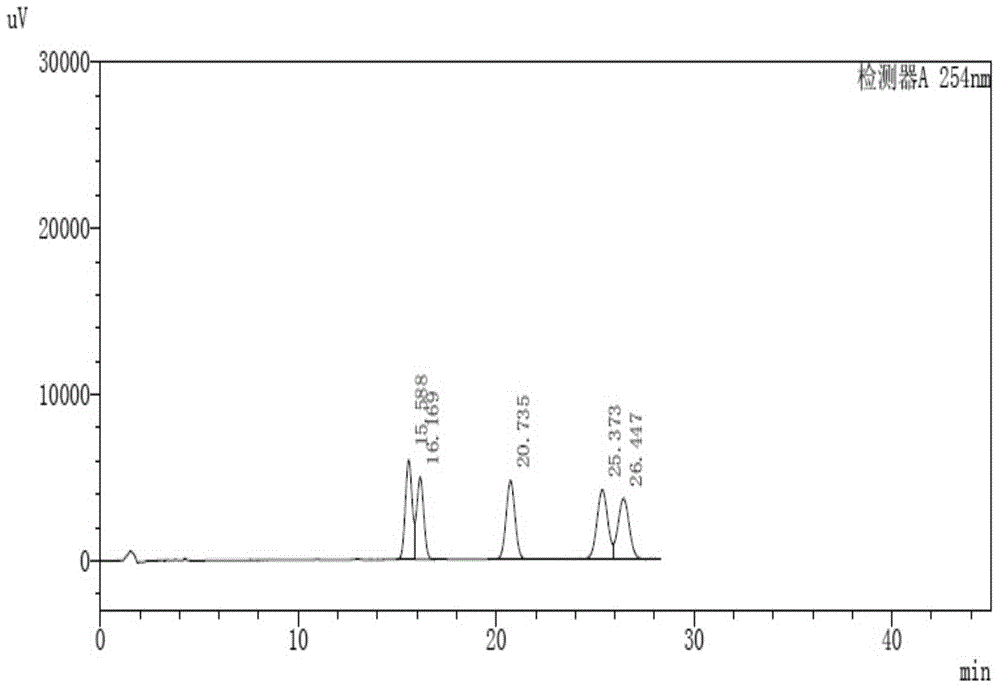
[0131] C, get the need testing solution of step a and the reference substance solution of step b respectively, inject high performance ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com