Total bufadienolides solid lipid nanoparticle drug delivery system for injection and preparation method thereof
A technology of solid lipid nanometer and total bufolide, which is applied to the digestive system, medical formulas, medical preparations of non-effective ingredients, etc., can solve problems such as poor intravenous infusion, redness and swelling at the infusion site, and improve biological Utilization, reducing vascular irritation, and ensuring drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~10
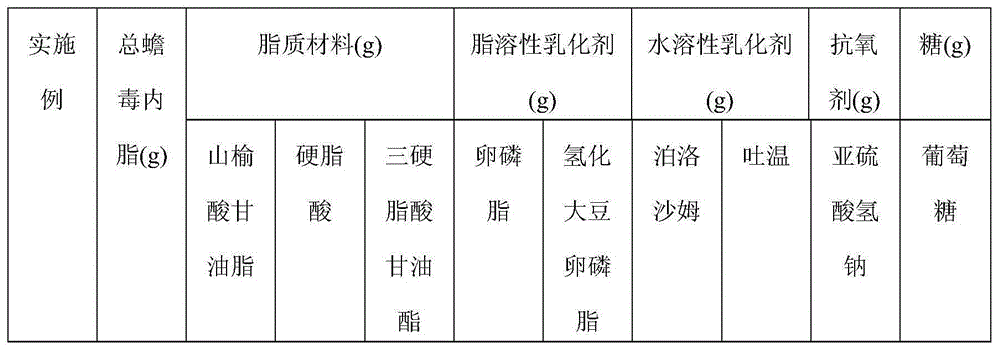
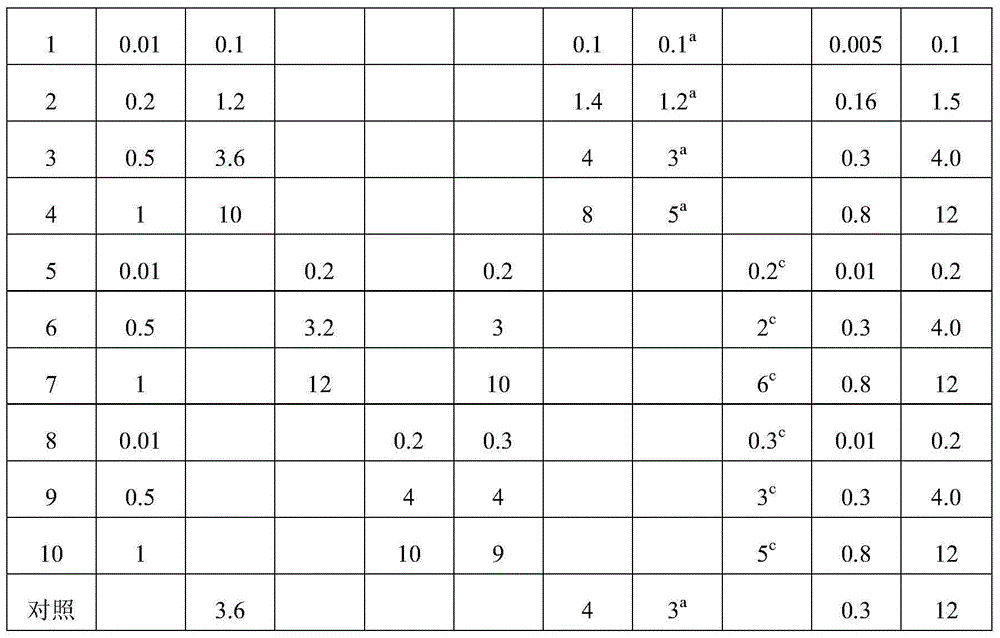
[0065] Examples 1-10 This example mainly describes the hot and high-pressure homogenization method. The components specified in Table 1 were prepared according to the following steps to obtain bufolide solid lipid nanoparticles of different embodiments.
[0066] (1) Heating the total toad toad lactone extract, lipid material and fat-soluble emulsifier to melting at the temperature shown in Table 2, to dissolve or disperse in the lipid to obtain a melt as an oil phase;
[0067] (2) Disperse the water-soluble emulsifier, antioxidant, and glucose in 100ml of water for injection to form a uniform water phase;
[0068] (3) Mix and stir the oil phase obtained in (1) and the water phase obtained in (2) at the temperature and rotating speed shown in Table 2 until uniform colostrum is formed;
[0069] (4) Transfer the colostrum prepared in (3) to a milk homogenizer, homogenize in a high-pressure milk homogenizer, and rapidly cool to room temperature or below to obtain total bufolide s...
Embodiment 11
[0078] The physical and chemical properties of the total bufolide solid lipid nanoparticles prepared in Examples 1-10 above were taken.
[0079] Take an appropriate amount of total bufotoxin solid lipid nanoparticle suspension, dilute it with water, then drop it on the copper grid covered with carbon film, stain with 2.0% sodium phosphotungstate negative staining solution, and observe the nanoparticles under a transmission electron microscope form and take photos.
[0080] As a result, the nanoparticles were observed under the electron microscope as spherical solid particles with uniform particle size and no aggregation and adhesion phenomenon.
Embodiment 12
[0082] The total bufolide solid lipid nanoparticles in the above-mentioned Examples 1-10 were taken to measure the encapsulation efficiency and drug loading capacity.
[0083] Total bufolide solid lipid nanoparticles and free drug were separated by sephadex glucose gel column chromatography, and the encapsulation efficiency was determined. Draw 1 ml of the nanoparticle suspension onto the column, and use distilled water as the elution medium for elution. Collect the eluate with opalescent part, quantitatively absorb this part of the eluate, add methanol to dissolve the nanoparticles; take another 1 ml of nanoparticle suspension, add methanol to dissolve. The encapsulation efficiency and drug loading were determined by HPLC.
[0084] The particle size, encapsulation efficiency and drug loading of total bufolide solid lipid nanoparticles measured in Examples 11-12 are shown in the following table:
[0085] table 3
[0086] Example Particle size (nm) Encapsulation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com