Valnemulin prodrug as well as preparation method and detection method thereof
A technology of warnemulin and prodrug, which is applied in the field of warnemulin prodrug and its preparation, can solve the problems of no structure, large molecular weight, and difficult analysis of nuclear magnetic spectrum, etc., and achieve good stability and drug loading capacity precise effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
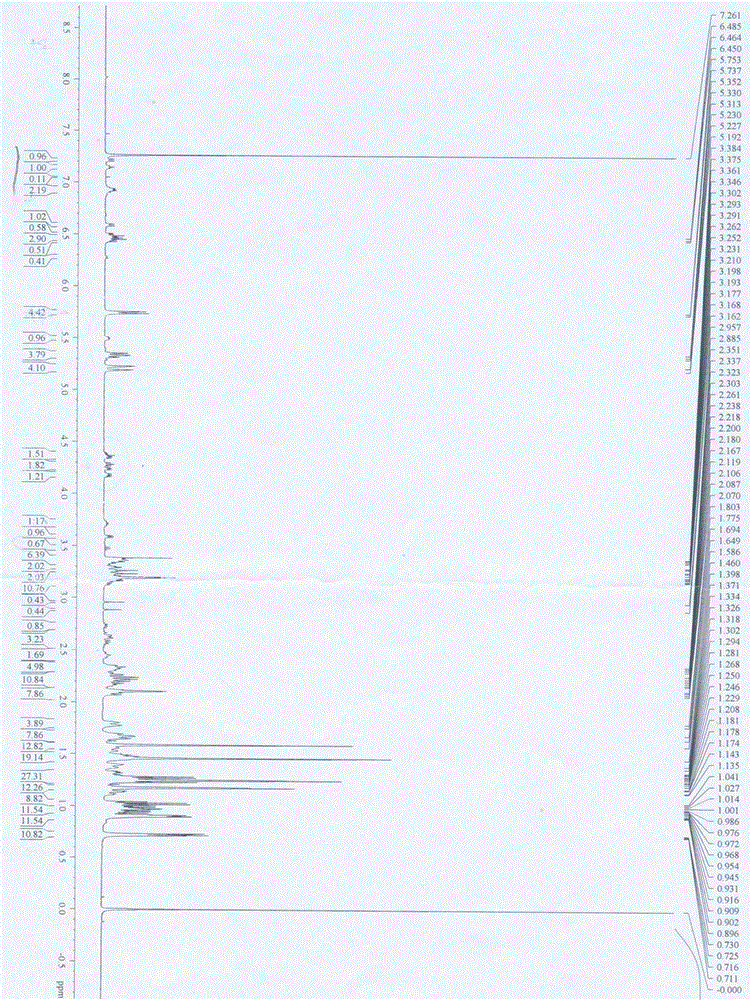
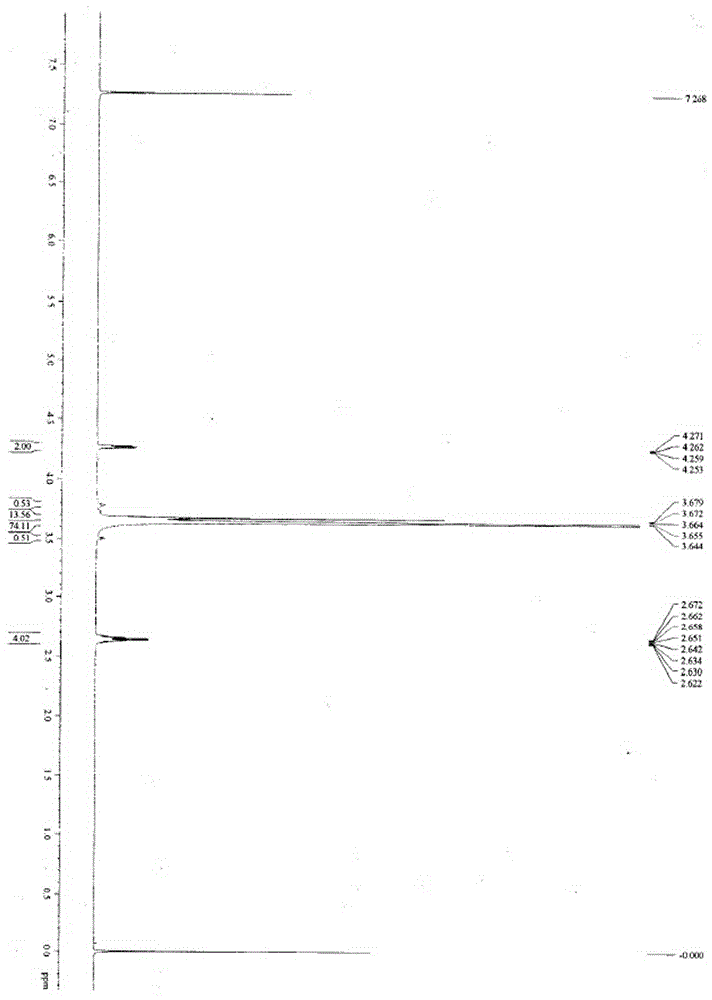
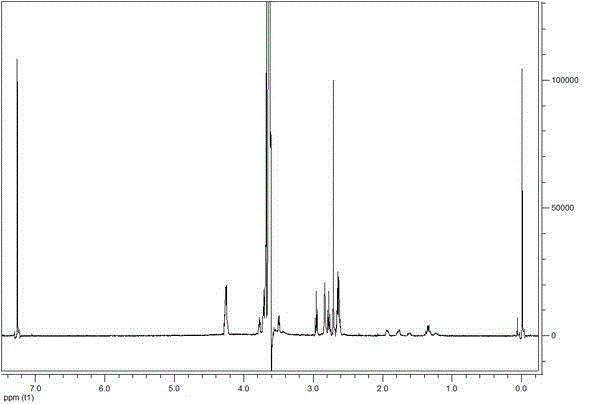
Image
Examples
Embodiment 1
[0059] Embodiment 1 The preparation method of ethylene glycol monomethyl ether-Varnemulin
[0060] The chemical reaction formula is as follows:
[0061] (1) Synthesis of ethylene glycol monomethyl ether-succinic acid:
[0062]
[0063] In a 50 mL three-necked flask, add ethylene glycol monomethyl ether (6.08 g, 0.08 mol), stir magnetically, add succinic anhydride (6.4 g, 0.064 mol), add 30 mL of toluene, stir fully to dissolve it, and heat Reaction at 80 °C for 4 h, monitored by TLC (developing solvent: PE:EA=5:1). After the reaction was completed, toluene was removed by distillation under pressure, and saturated NaHCO was added 3 Solution (50 mL) was adjusted to pH=8, ethyl acetate (20 mL×3) was used to extract impurities, the aqueous phase was taken, adjusted to pH 2~3 with 1 mol / L hydrochloric acid, dichloromethane (20 mL×4) was extracted, and the organic phase Wash with saturated brine until neutral, reverse extraction with water, dry the organic phase by adding anhy...
Embodiment 2
[0074] The preparation method of embodiment 2 warnemulin prodrug
[0075] (1) Synthesis of PEG derivatives with carboxyl groups at the end:
[0076]
[0077] Add PEG (molecular weight: 2000) (20 g, 10 mmol), toluene (80 mL), and dry pyridine (2 mL) into a 250 mL three-neck flask, stir magnetically and heat to 60°C to dissolve, and succinic anhydride (2.5 g, 25 mmol) was dissolved in toluene (20mL) at 60°C, and the dissolved succinic anhydride was slowly dropped into the dissolved PEG or mPEG, and reacted at 60°C for 1h, and the temperature was raised to 80°C for 36h . After the reaction was completed, the temperature was naturally lowered, the solvent was removed by rotary evaporation, and saturated NaHCO was added to the residue. 3 The solution (60 mL) was stirred for 30 min, and a white milky substance was produced during the addition, filtered through a Buchner funnel, and the filtrate was extracted with ethyl acetate (20 mL×5), the water phase was taken, cooled to 0-5...
Embodiment 3-7
[0126] Embodiment 3-7 Warnemulin prodrug and preparation method thereof
[0127] Embodiments 3-7 are respectively a warnemulin prodrug and its preparation method, which are similar to those in Example 2, except that the PEG or mPEG modifiers are different and the technical parameters involved are different, see below for details surface:
[0128]
[0129]
[0130]
[0131]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com