Rapid high-selectivity hypochloric acid fluorescence probe and application thereof
A technology of hypochlorous acid and hypochlorite, applied in fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., can solve problems such as slow response speed, poor selectivity, complex synthesis, etc., and achieve good stability, good water solubility, and synthetic simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] (Scheme 1) Dissolve 285.5mg (1.0mmol) of N-butyl 4-chloro-1,8-naphthalimide, 220mg (2.0mmol) of hydroquinone and 552mg (4.0mmol) of potassium carbonate in 10mL of dimethyl base sulfoxide (DMSO), and then reacted at a constant temperature of 80°C for 10h. The reaction solution was poured into 100 mL of water, extracted with dichloromethane to obtain a dichloromethane phase, and then rotary evaporated to obtain a crude product, which was separated by column chromatography using a dichloromethane system to obtain 256 mg of a yellow pure product with a yield of 71%.
[0037] (Scheme 2) Dissolve 285.5 mg (1.0 mmol) of 4-chloro-1,8-naphthalimide, 330 mg (3.0 mmol) of hydroquinone and 828 mg (6.0 mmol) of potassium carbonate in 10 mL of dimethylsulfoxide ( DMSO), then reacted at a constant temperature of 80 °C for 10 h, poured the reaction solution into 100 mL of water, extracted with dichloromethane to obtain a dichloromethane phase, and then rotary evaporated to ...
Embodiment 2
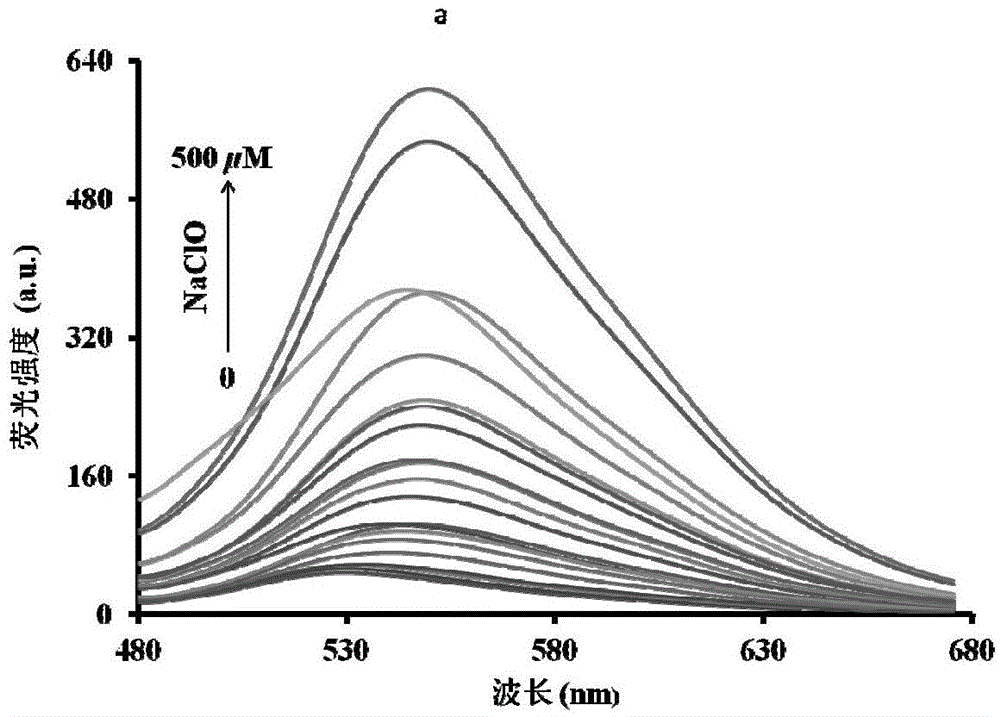
[0043] The inventor of the present invention has carried out following test: (a) the influence of different concentration NaClO (0~500 μ M) on probe (5 μ M) fluorescence spectrum; linear relationship between. The above determination is carried out in a mixed system of absolute ethanol and water (5:5, v / v), 5mM PBS, pH 7.4, the probe used is the probe prepared in Example 1, and all spectra The tests are all measured after adding NaClO for 10 minutes at 25°C. See Figure 1 for the results.
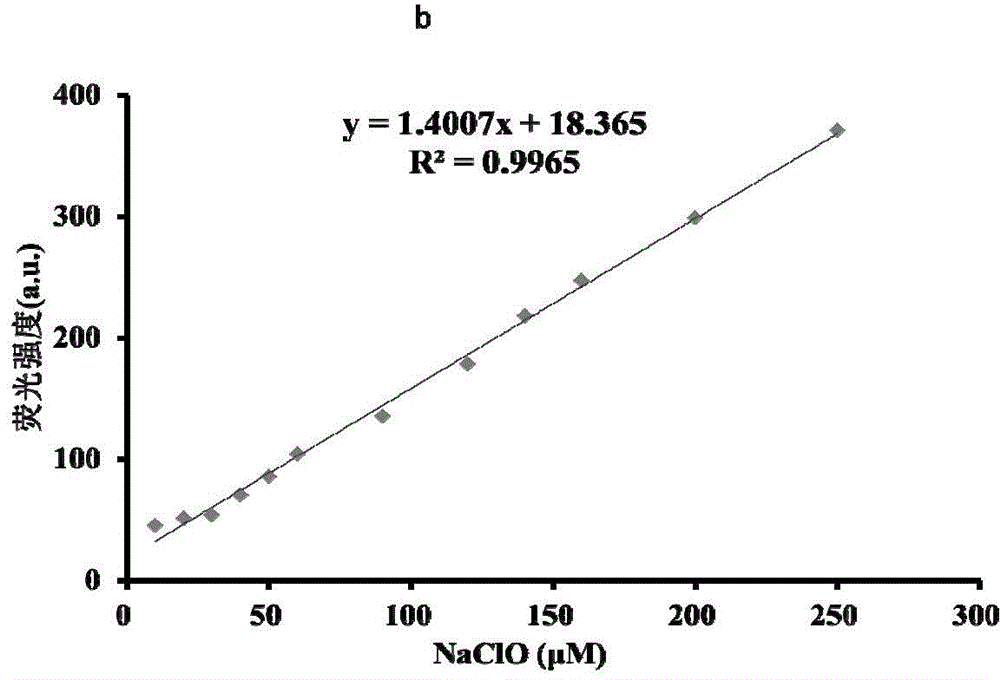
[0044] It can be seen from Figure 1 that with the increase of NaClO concentration in the probe solution, the fluorescence spectrum gradually increases, and within the concentration range of 0-250 μM NaClO, the concentration of NaClO and the fluorescence intensity have a linear relationship. Therefore, the NaClO in the sample can be quantitatively analyzed by means of the probe of the present invention.
Embodiment 3
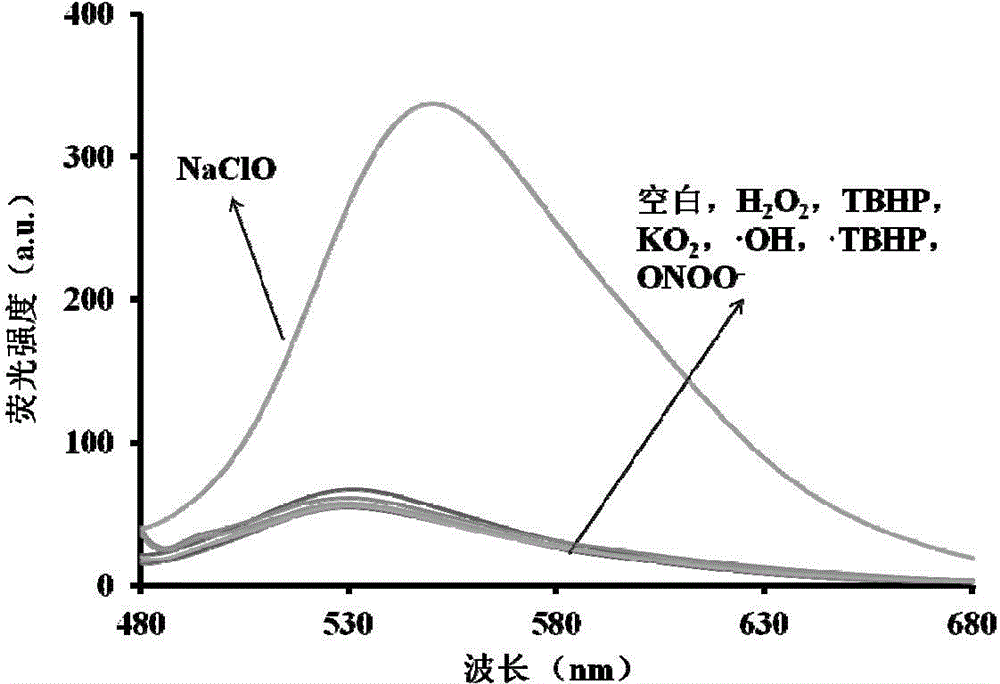
[0046] Effect of different analytes (40 μM) on the fluorescence spectra of probes (5 μM). Analytes include: Hydrogen peroxide H 2 o 2 , tert-butanol peroxide TBHP, potassium superoxide KO 2 , hydroxyl radical · OH, peroxide tert-butanol radical · TBHP, peroxynitrite ONOO - , sodium hypochlorite NaClO, and their concentrations are all 40 μM. All test conditions are carried out in the mixed system (5:5, v / v) of absolute ethanol and water, 5mM PBS, pH 7.4, the probe used is the probe prepared in embodiment 1, and all Spectral tests were all measured after adding NaClO for 10 min at 25°C. Specifically, pipette 50 μL of the probe stock solution (1 mM) into a 10 mL colorimetric tube, then add 5 mL of absolute ethanol, then pipette 20 μL of the above analyte stock solution (10 mM) into the colorimetric tube, and then pipette 0.5 mL of PBS solution (pH7.4, 100mM), and finally dilute to 10mL with ultrapure water. Shake well, let it stand for 10min, then measure. The result is as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com