3-vinyl-quinoxaline-2(1H)-one derivatives acting on FGFR-1, preparation method and uses thereof
A technology of FGFR-1 and methylquinoxaline, which is applied to medical preparations containing active ingredients, drug combinations, and pharmaceutical formulas, etc., can solve problems such as poor selectivity, low affinity, weak inhibition strength, etc., and achieve good safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the synthesis of compound
[0037]
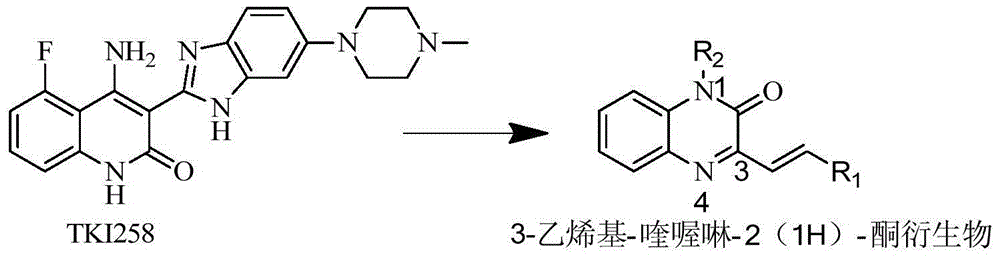
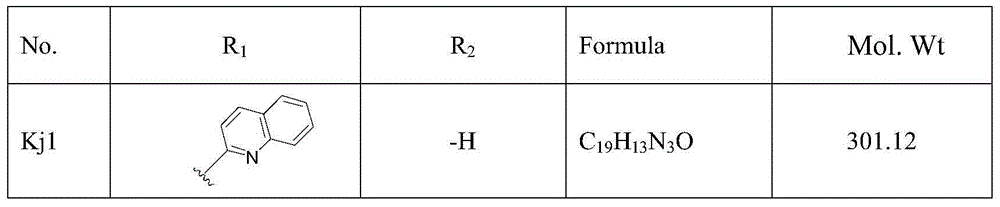
[0038] It can be seen from 1 and 2 that 1 is the synthesis route of compounds Kj1, Kj4, Kj5, and Kj6 of the present invention and 2 is the synthesis route of compounds Kj2 and Kj3. Wherein i: pyruvic acid, n-butanol, reflux; ii: acetic anhydride, piperidine, reflux; iii: anhydrous acetone, benzyl bromide, anhydrous potassium carbonate, reflux; iv: pyruvic acid, ethanol; v: acetic anhydride , piperidine, reflux.
[0039] 1, Synthesis of 3-methylquinoxalin-2(1H)-one
[0040] O-phenylenediamine (1.00 g, 9.25 mmol) and pyruvic acid (11 mmol) were dissolved in hot n-butanol (35 mL) and heated to reflux. The reaction was stirred for 3 hours, and a large amount of flocculent products precipitated out, and the mixture was cooled to room temperature. Suction filtration, washing with n-hexane 2-3 times to obtain crude 1-hydrogen 3-methylquinoxalin-2(1H)-one. The crude product was purified by recrystallization from ethanol ...
Embodiment 2
[0057] Example 2 Antitumor Activity Detection of Compounds
[0058] MTT is a yellow compound that accepts hydrogen ions. It can act on the respiratory chain in the mitochondria of living cells. The succinate dehydrogenase in the mitochondria of living cells reduces it to produce a water-insoluble blue-purple crystalline formazan. And deposited in cells, and dead cells do not have this function. DMSO can dissolve formazan in cells, and use an enzyme-linked immunosorbent assay to measure its light absorption value at a specific wavelength. Within a certain range of cell numbers, the amount of formazan formed is proportional to the number of cells, so it can indirectly reflect the number of living cells. quantity.
[0059] Cell culture: H460, Hct116, and normal liver cells HL7702 were cultured in RPMI 1640 medium containing 10% fetal bovine serum; Hela229 and B16-F10 were cultured in DMEM high-glucose medium containing 10% fetal bovine serum. The culture conditions were 37°C, 5...
Embodiment 3
[0072] Example 3 Kinase inhibitory activity assay
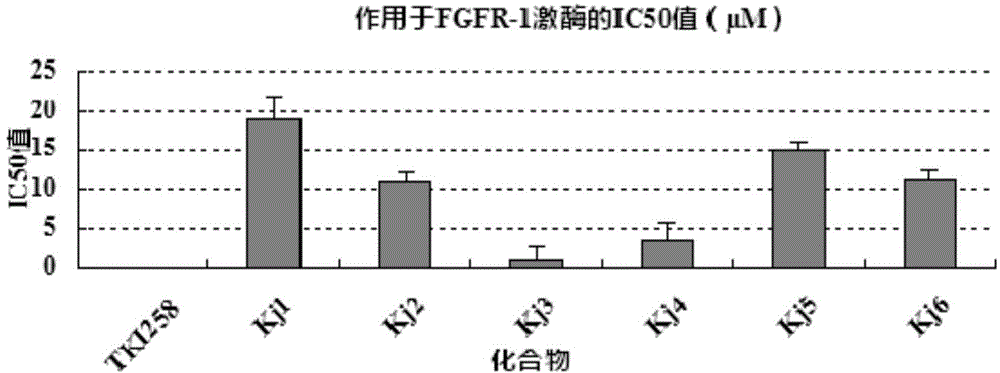
[0073] Using LANCE Ultra TR-FRET Assays, the FGFR-1 inhibitory activity of the six synthesized compounds was detected in vitro, and TKI258 was used as a positive control to test the IC of the compounds acting on FGFR-1 kinase 50 value.
[0074] Inhibition experiment of synthesized compounds on FGFR-1 kinase
[0075] (1) Prepare 1x kinase reaction buffer (50mM HEPES, pH 7.5; 0.01% Tween-20; 10mM MgCl2; 2mM DTT; 1mMEGTA), 1x LANCE Detection Buffer and kinase reaction termination solution (40mM EDTA).
[0076] (2) Preparation of compounds for kinase experiments: final dilution of compounds to 400 μM, 40 μM, 4 μM, 0.4 μM and 0.04 μM with 100% DMSO and 1× kinase buffer.
[0077] (3) Kinase reaction: Add FGFR-1 enzyme to 1x kinase buffer to prepare 4x enzyme solution. Prepare 2x peptide solution by adding ATP and ULight-JAK-1peptide to 1x kinase buffer. Add 2.5 μl 4x enzyme solution prepared above to each well and incubate at roo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com