Congenital heart disease transcatheter closure device
A congenital heart disease, blocking device technology, applied in the field of medical devices, can solve the problems of difficult to determine the long-term toxic and side effects of metals, chronic vascular damage, mechanical stimulation, etc., to eliminate toxic side effects, reduce metal toxic side effects, and avoid damage. and stimulating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] The present invention will be described in further detail below in conjunction with the accompanying drawings and specific embodiments.
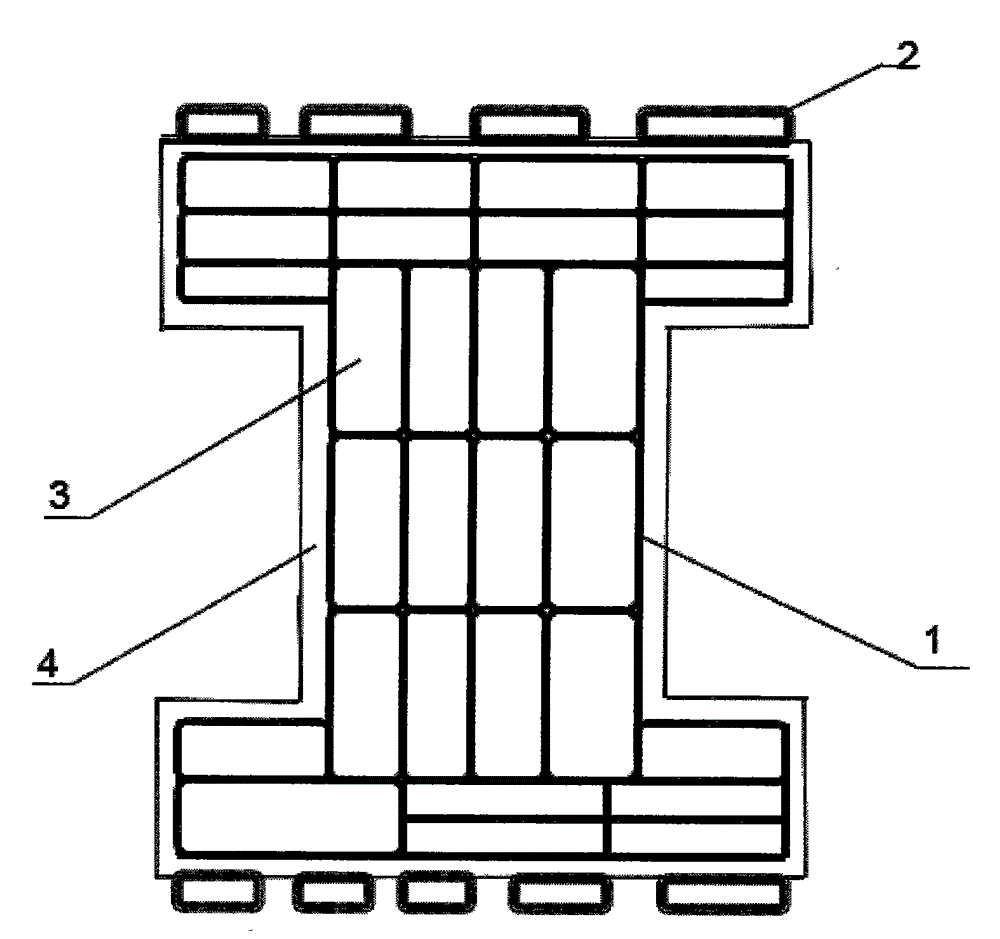
[0012] Depend on figure 1 It can be seen that a congenital heart disease blocking device comprises an I-shaped bracket 1 made of a nickel-titanium alloy wire mesh with a diameter of 0.0031-0.0033 inches, and the two outer sides of the upper and lower ends of the I-shaped bracket 1 are provided with Several bumps 2, the bumps can increase the contact with the body tissue; polyester fiber 3 is filled in the I-shaped bracket 1, and a layer of polyurethane foam 4 is coated on the metal wire of the I-shaped bracket 1.
[0013] In the present invention, the preferred material used for the I-shaped stent 1 is TiNi shape memory alloy, and its diameter is any size between 0.0031-0.0035 inches. The alloy has superelastic properties, and its fatigue resistance and corrosion resistance are also much better than stainless steel. Using TiNi shape ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com