Recombinant oncolytic II-type herpes simplex virus (HSV) and pharmaceutical composition thereof
A herpes simplex virus and oncolytic technology, applied in the field of recombinant herpes simplex virus, can solve the problems of tumor-associated antigen capture and presentation, and achieve the effect of enhancing immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] A method for preparing recombinant oncolytic herpes simplex virus type II, comprising the following steps:
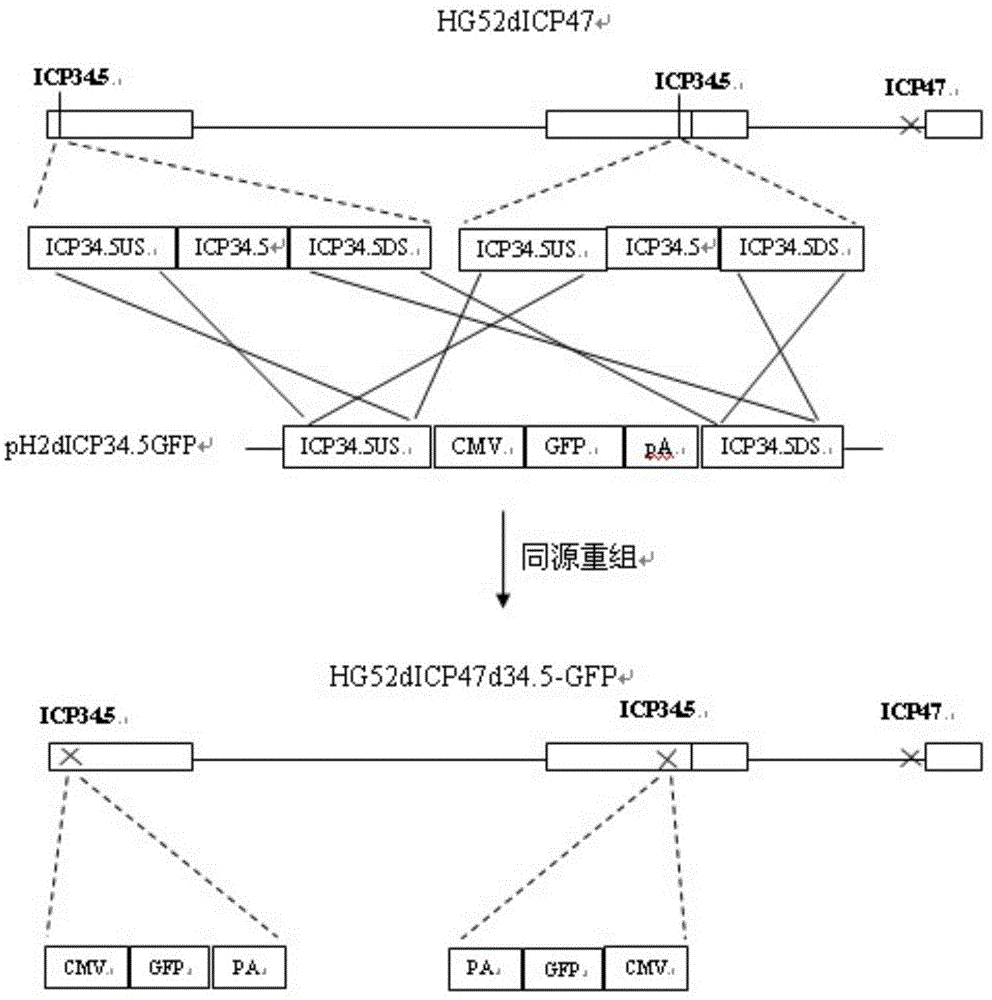
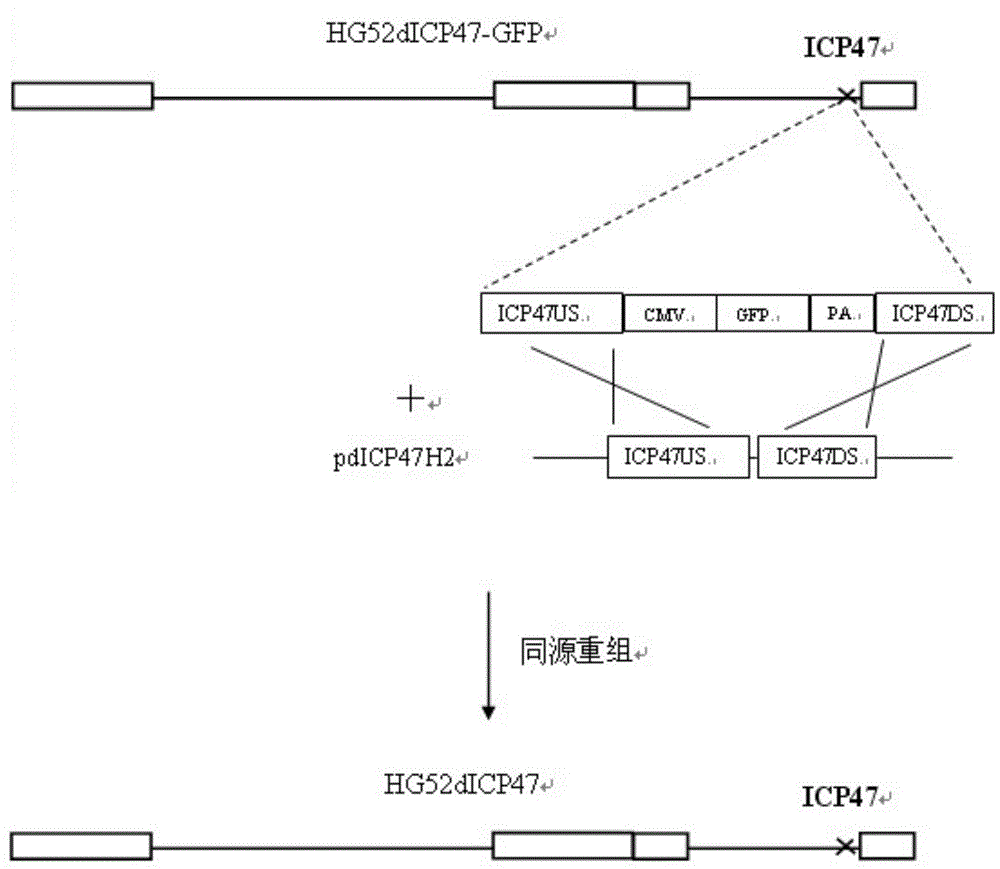
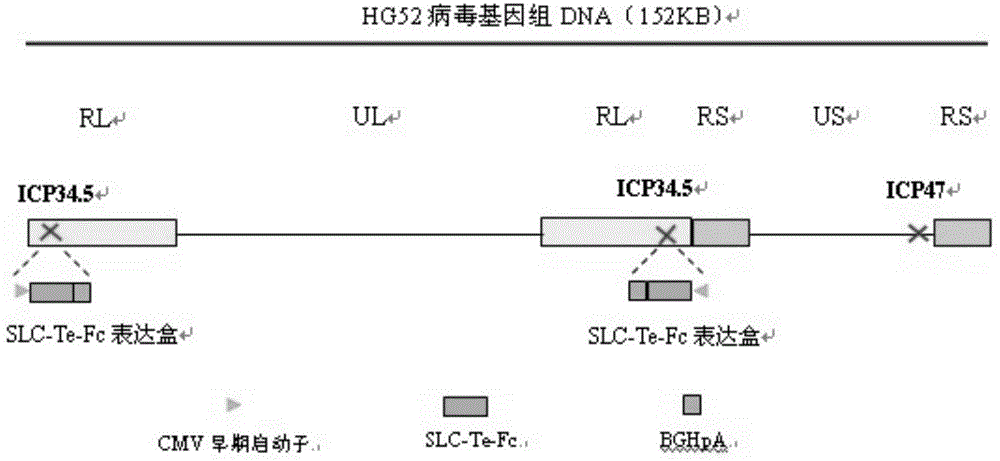
[0078] (1) Delete the ICP47 gene from the wild type II herpes simplex virus HG52 strain to construct HG52dICP47 recombinant type II herpes simplex virus:
[0079] a. Genomic DNA of type II herpes simplex virus HG52 strain is extracted;
[0080] b. Construct the plasmid pdICP47H2 containing the upstream flanking region sequence and the downstream flanking region sequence of the ICP47 gene:
[0081] b1. Use the following primers for amplifying the sequence of the upstream flanking region of the ICP47 gene and the sequence of the downstream flanking region of the ICP47 gene, and use the genomic DNA obtained in step a as a template to amplify the sequence of the upstream flanking region and the downstream flanking region of the ICP47 gene by PCR sequence;
[0082] Amplify ICP47 gene forward primer 146554 AGAGTCACGACGCATTTGCCC 146574
[0083] Upstream flanking se...
Embodiment 2
[0127] Example 1 is used to prepare virus preparation medicine:
[0128] 1. Establishment of two tumor animal models and the in vivo oncolytic effect of the recombinant virus vector of the present invention
[0129] 1) Purchase C57 / BL and Balb / c female mice from the Experimental Animal Center of the Chinese Academy of Medical Sciences, 4-6 weeks old (C57 / BL is a tumor-bearing strain of melanoma B16R, and BALB / c is a tumor-bearing strain of CT26) , 16-20 grams;
[0130] 2) Node melanoma (B16R) and intestinal cancer (CT26) cells were selected respectively, and inoculated subcutaneously into the right axilla of the mouse with a trocar. Inject 10 subcutaneously in the flank of each mouse 5 Tumor cells, when the diameter of the tumor mass was about 0.5-0.7 cm (5-7 days after injection), randomly divided into 3 groups with 10 animals in each group (the experimental group was oHSV2-CADV, and the control group was pSLC-Te-Fc DNA vaccine, blank control group),
[0131]3) Then use t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com