Production method of finished-product tissue engineered bone
A technology of tissue engineering and finished products, applied in medical science, prosthesis, etc., can solve the problems that the sterility of tissue-engineered bone cannot be well guaranteed, the construction period takes a long time, and rejection reactions, etc., to facilitate long-term storage , high integrity, and improved safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
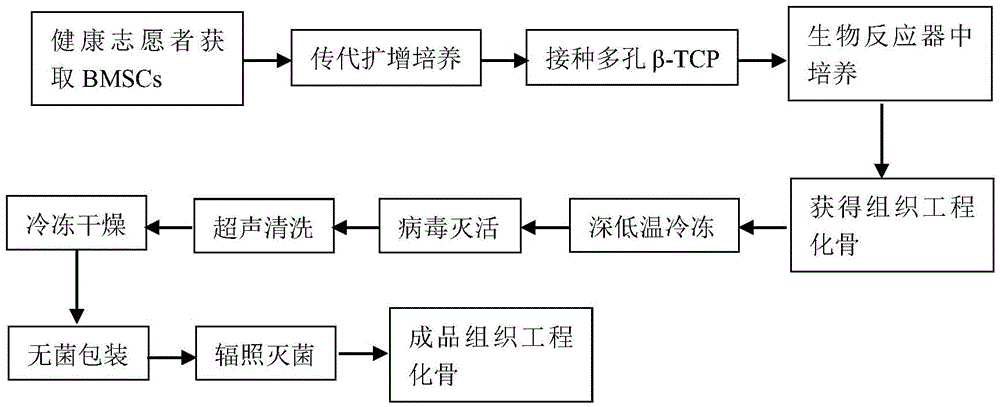
[0033] Such as figure 1 as shown,
[0034] (1) Firstly, the bone marrow was extracted from the iliac crest of volunteers, and bone marrow mononuclear cells were obtained by adherent culture method, and expanded and cultured. The flow cytometry method detects the marker antigen on the cell surface, which can confirm that the obtained cells are bone marrow mesenchymal stem cells. The third to sixth generation cells were applied to the construction of tissue engineered bone in vitro.
[0035] (2) The cells obtained by subculture were made into a single cell suspension, and the cell concentration was controlled at 2.0×10 6 / ml or so. Then infiltrate the porous β-TCP scaffold into the single cell suspension, and use the method of vacuuming (vacuum degree is 25.33Kpa) to make the single cell suspension fully enter the interior of the porous scaffold. Cultivate in the cell incubator for 2 hours to obtain the cell / scaffold complex, then inoculate the cell / scaffold complex into the...
Embodiment 2
[0045] Such as figure 1 as shown,
[0046] (1) Firstly, the bone marrow was extracted from the ilium of volunteers, and the bone marrow mononuclear cells were obtained by density gradient centrifugation, and then expanded and cultured. The third to sixth generation cells were applied to the construction of tissue engineered bone in vitro.
[0047] (2) The cells obtained by subculture were made into a single cell suspension, and the cell concentration was controlled at 2.0×10 7 / ml. Then the porous hydroxyapatite scaffold is soaked into the single cell suspension, and the single cell suspension is fully entered into the interior of the porous scaffold by vacuuming, and the vacuum degree is 75.99Kpa. Then cultivate in the cell culture incubator for 4 hours to obtain the cell / scaffold complex, inoculate the cell / scaffold complex into the perfusion bioreactor, and add the medium After standing for 4 hours, start the perfusion bioreactor , the cell / scaffold complex was continuou...
Embodiment 3
[0057] Such as figure 1 as shown,
[0058] (1) Firstly, the bone marrow was extracted from the ilium of volunteers, and the bone marrow mononuclear cells were obtained by density gradient centrifugation, and then expanded and cultured. The third to sixth generation cells were applied to the construction of tissue engineered bone in vitro.
[0059] (2) The cells obtained by subculture were made into a single cell suspension, and the cell concentration was controlled at 2.0×10 7 / ml. Then soak the porous coral support into the single-cell suspension, and use a vacuum method to make the single-cell suspension fully enter the inside of the porous support, and the vacuum degree is 55Kpa. Then cultivate in the cell culture box for 3 hours to obtain the cell / scaffold complex, inoculate the cell / scaffold complex into the perfusion bioreactor, and add the culture medium to start the perfusion bioreactor after standing for 3 hours, The cell / scaffold complex was continuously perfused...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com