A kind of Glypican GPC3 protein fragment and its application and hybridoma cell line prepared
A hybridoma cell line and cell line technology are applied in the field of monoclonal antibody hybridoma cell line GPC3-1H5 to achieve the effects of reducing production cost, improving sensitivity and accuracy, and high titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 gpc 3 Cloning of genes and construction of recombinant expression plasmids
[0030] To contain gpc The plasmids of the 3 genes were used as templates for PCR amplification.
[0031] Upstream primer: 5'-CGGAATTCCTTGGTGGTGGCGATGCT-3', inserted into the EcoRI restriction site.
[0032] Downstream primer: 5'-CGGGATCCCCCGAGGTTGTGAAAGGT-3', inserted into the BamHI restriction site.
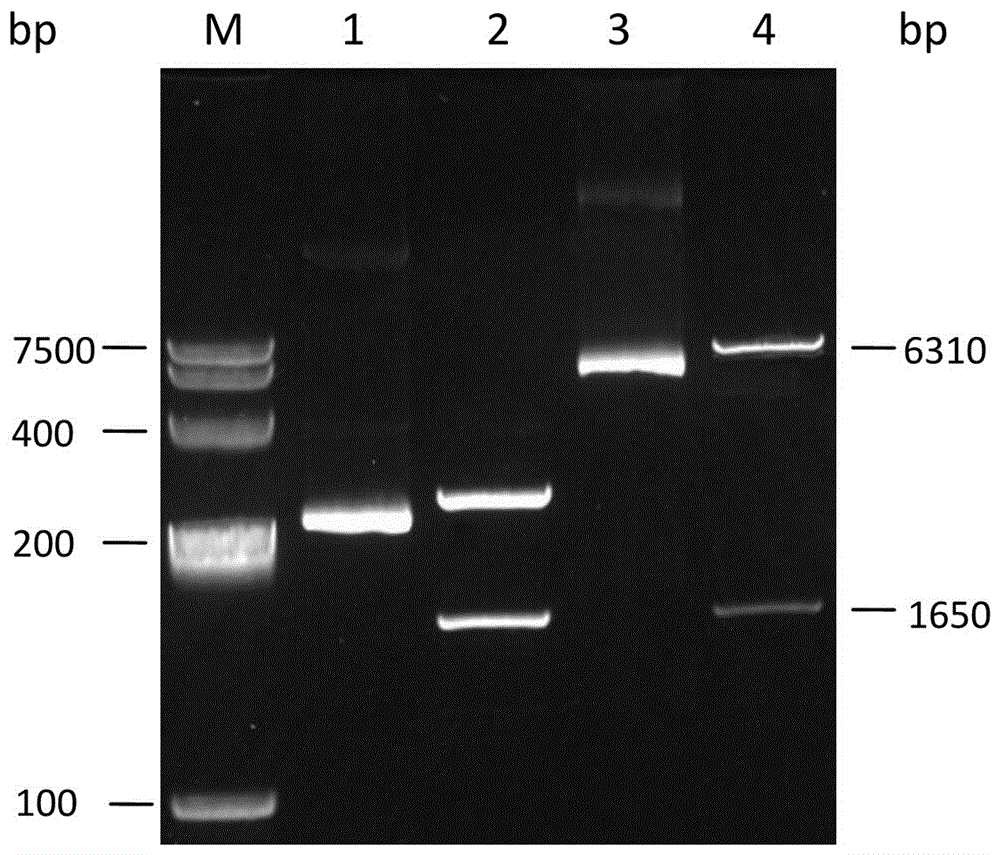
[0033] Amplification conditions: pre-denaturation at 95°C for 5 min; denaturation at 94°C for 1 min, annealing at 62°C for 1 min, extension at 72°C for 2 min, a total of 30 cycles; extension at 72°C for 10 min. The PCR products were detected by agarose gel electrophoresis, and the experimental results were as follows: figure 1 As shown, the resulting 1650bp nucleotide fragment was amplified.
[0034] The PCR product was recovered after agarose gel electrophoresis, ligated into pMD18-T vector, and the product was ligated with T4 ligase in a water bath at 16°C. The ligation produ...
Embodiment 2
[0037] Induced expression and purification of embodiment 2 fusion protein
[0038] HEK293 cells in good condition were treated with 2×10 5 Cells / well were cultured in 6-well plates. After culturing for 24 hours, transfection was performed when the cell confluence was 80%-90%. Rinse 3 times with PBS, and add serum-free DMEM high-glucose medium. Follow Lipofectamine TM 2000 transfection reagent instructions, the recombinant expression plasmid CMV- gpc3Transfection was carried out, and a blank control group (HEK293 cells) and a positive control group (p3XFLAG-CMV-14) were set up at the same time. The DMEM high-glucose medium containing 0.5 mg / L G418 and 10% fetal bovine serum was used for pressurized screening, and the monoclonal cells were prepared by the limiting dilution method, and finally a cell line stably expressing the recombinant protein was obtained.
Embodiment 3
[0039] Example 3 Preparation of Monoclonal Antibody Hybridoma Cell Line GPC3-1H5
[0040] Amplify the cell line expressing the recombinant protein prepared by the above steps by conventional methods, collect the bacteria by centrifugation at low temperature, perform ultrasonic crushing in an ice bath, centrifuge at 4°C, 8000r / min for 20min, collect the supernatant, and purify according to Anti-FLAG resin Kit purification to obtain GPC3 recombinant protein.
[0041] The expressed GPC3 recombinant protein was purified as an antigen, and immunized according to the standard immunization procedure, that is, the first immunization was mixed with equal volume of recombinant protein and complete Freund's adjuvant, intraperitoneally injected into mice, the dose was 100ul / mouse, which contained recombinant protein 50ug. From the second immunization, mix the same volume of recombinant protein and incomplete Freund's adjuvant, the dose of recombinant protein immunization is the same as t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com