Berberine hydrochloride self-microemulsion preparation having good oral bioavailability and preparation method thereof
A technology for berberine hydrochloride and an emulsion preparation is applied in the field of berberine hydrochloride self-microemulsion preparation and its preparation, which can solve the problem of not being able to ensure that berberine hydrochloride is not metabolized in the intestinal tract and the like, and achieves the improvement of oral bioavailability and the avoidance of The effect of intestinal metabolism, carrying and taking convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 berberine hydrochloride self-microemulsion preparation
[0029] 1. Prepare the following components in mass ratio: 3-5% berberine hydrochloride, 3-5% penetration enhancer, 40-50% oil phase, 30-40% emulsifier and 10-20% co-emulsifier.
[0030] Wherein, the penetration enhancer is sodium caprate or sodium oleate; the oil phase is isopropyl myristate, isopropyl palmitate, peppermint oil, rose oil or lemon oil. The emulsifier is polyoxyethylene ether-40 hydrogenated castor oil, polyoxyethylene 10 oleyl ether, poloxamer PF127 or poloxamer PF68; the co-emulsifier is ethanol or glycerin.
[0031] 2. Preparation:
[0032] S1. according to the formula described in claim 1, berberine hydrochloride, oil phase, emulsifier and co-emulsifier are mixed, heated and stirred to obtain clear solution, i.e. pre-microemulsion;
[0033] S2. Add the pre-microemulsion obtained in S1 into distilled water at 37° C. and stir slightly to form a self-microemulsion.
Embodiment 2
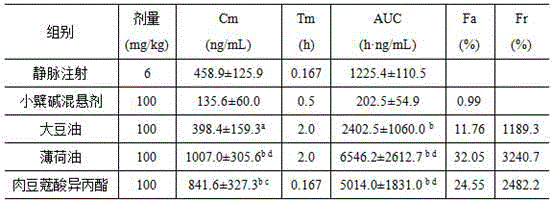
[0034] Example 2 SD rat bioavailability experiment
[0035] 1. Dosing regimen
[0036] Healthy SD rats (200 ± 20 g) were given different cinnamon preparations (100 mg / kg), and intravenous (intravenous, iv) injection (6 mg / kg) was used as the control preparation, before and after administration, respectively At 0.167 h, 0.5 h, 1.0 h, 1.5 h, 2.0 h, 4.0 h, 6.0 h, 8.0 h, 10 h, and 12 h, 0.40 mL of blood was taken from the orbital vein, and the blood samples were processed according to the assay method, and the blood drug concentration was determined by HPLC .
[0037] 2. Blood sample processing
[0038] Take 0.45 mL of blood from SD rats and put it in a centrifuge tube treated with sodium heparin, centrifuge at 3500 rpm for 15 min, take 0.15 mL of supernatant plasma, add 0.30 mL of acetonitrile to precipitate protein, vortex for 3 min, then centrifuge at 10000 rpm for 10 min, take The supernatant was passed through a 0.22 μm filter membrane, and the BBR content was determined...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com