Epidermal anesthesia gel and preparation method thereof
A gel and epidermis technology, which is applied in anesthetics, aerosol delivery, liquid delivery, etc., can solve problems such as pH value hindrance and difficulty in coating, and achieve the effects of fast onset of action, reduced drug burden, and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
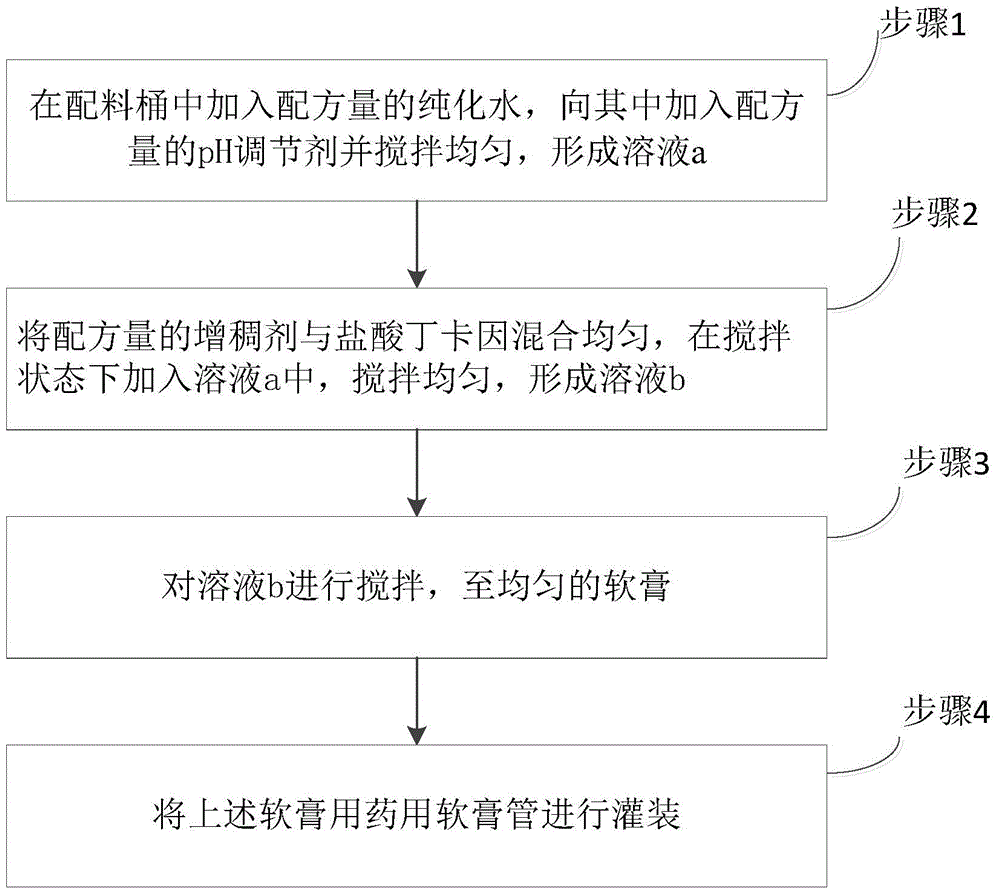
Image
Examples
Embodiment 1
[0021] On the basis of the above, the thickener is specifically xanthan gum, and the pH regulator is specifically sodium hydroxide. In the formula of the epidermal anesthesia gel in the present invention, the weight percentage of each component is: tetracaine hydrochloride, 4.7 parts ; Xanthan gum, 2 parts; Sodium hydroxide, 0.6 parts; Purified water, 92.7 parts.
[0022] The epidermis anesthesia gel in the present invention can replace similar imported drugs, and has better effect, reduces the drug burden of Chinese people, has fast onset, small side effects, and wide application range. After a single administration, the anesthesia effect on most patients Up to 4-6 hours, long-term action. In this formula, xanthan gum is used as a thickener, which is easy to coat, and the pH is easy to control during the preparation process, easy to repeat, and the preparation process is stable. If sodium carboxymethyl cellulose is used as a thickener, the The gel is not uniform, it is diffi...
Embodiment 2
[0024] On the basis of the above, the thickener is specifically xanthan gum, and the pH regulator is specifically sodium hydroxide. In the formula of the epidermal anesthesia gel in the present invention, the weight percentage of each component is: tetracaine hydrochloride, 3 parts ; Xanthan gum, 1 part; Sodium hydroxide, 0.7 part; Purified water, 95.5 parts.
Embodiment 3
[0026] On the basis of the above, the thickener is specifically xanthan gum, and the pH regulator is specifically sodium hydroxide. In the formula of the epidermal anesthesia gel in the present invention, the weight percentage of each component is: tetracaine hydrochloride, 5 parts ; Xanthan gum, 3 parts; Sodium hydroxide, 0.5 parts; Purified water, 91.5 parts.
[0027] In this example, 5 parts of tetracaine hydrochloride are used. Generally, when the amount of tetracaine hydrochloride exceeds 4.7 parts, the absorption of tetracaine hydrochloride by the human body will increase, and the onset time and analgesic effect will not be affected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com