A kind of compound antidiarrheal lignan a with anti-drug resistance activity and use thereof
A technology of antidiarrheal lignans and compounds, applied in the field of medicine, can solve problems such as difficulty in drug-resistant bacteria and increased mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The antidiarrheal muzi 20Kg was extracted 3 times with 10 times the volume fraction of 75% ethanol-water solution by heating and refluxing for 1 hour each time. The extract was filtered and the ethanol was recovered under reduced pressure to obtain the extract. The extract part (1009) was subjected to repeated silica gel adsorption column chromatography, and gradient elution was carried out with dichloromethane-methanol (100:1-1:100) system, and Sephadex LH-20 column chromatography was carried out with methanol-water (10:1-1:100). :10), dichloromethane-methanol (5:1-1:1) or pure methanol for elution, HPLC with methanol-water (5:95-95:5) system for gradient elution, combined with recrystallization, etc. Means of separation and purification to obtain antidiarrheal lignan A.
Embodiment 2
[0028] (1) The antidiarrheal Muzi extract was processed by HPD600 macroporous resin column chromatography, followed by gradient elution with water and volume fractions of 60% ethanol, 70% ethanol, 80% ethanol, 90% ethanol and 100% ethanol;
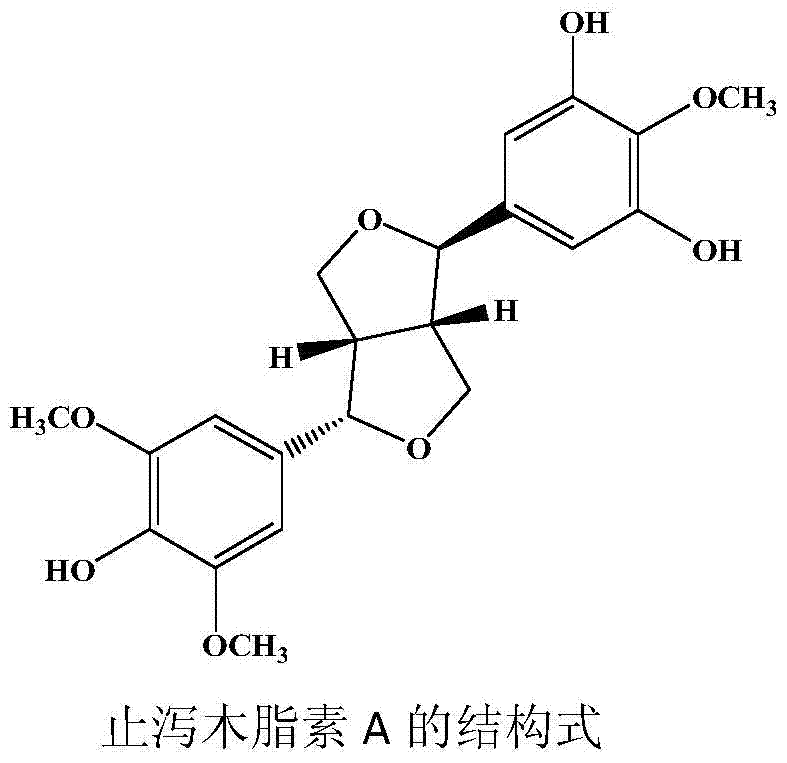
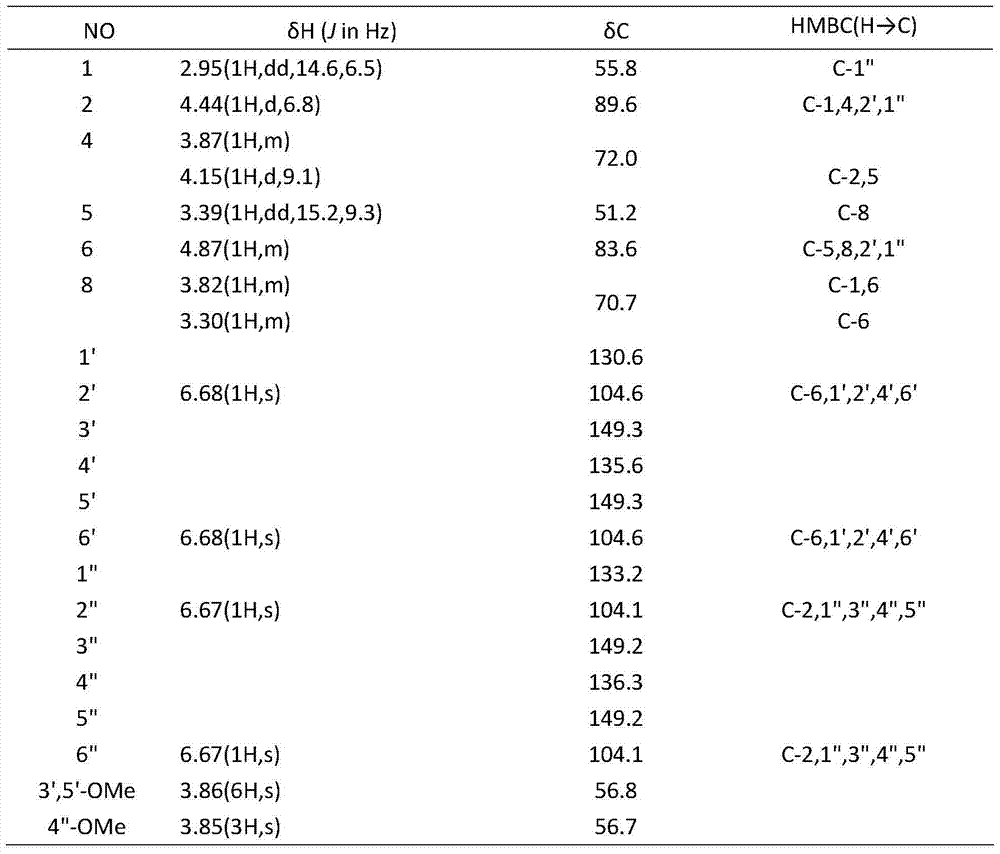
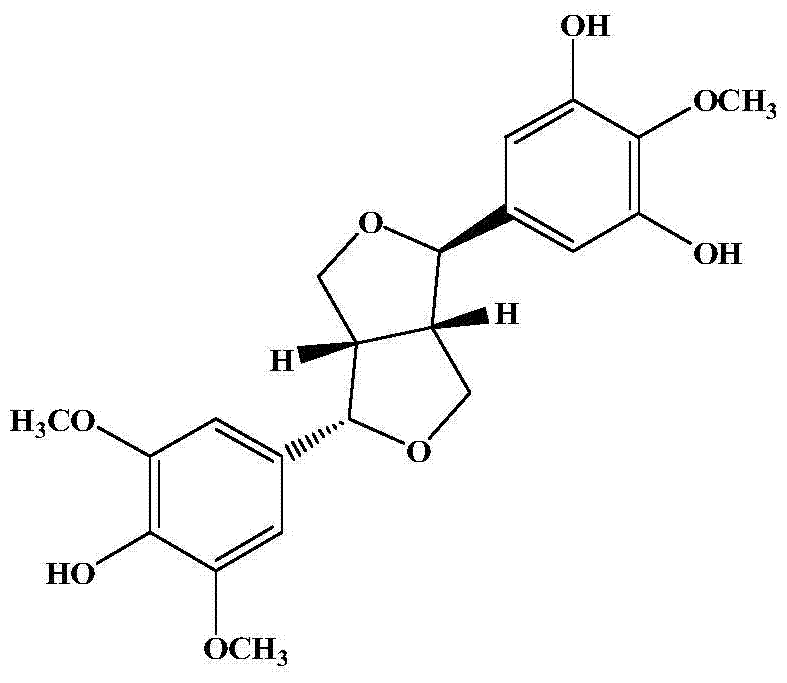
[0029] (2) The volume fraction obtained in step (1) is 80% ethanol. The eluate is separated by silica gel column chromatography and eluted with a gradient of dichloromethane: methanol 100:1, 100:4, 100:8, 100:16 (volume mixing ratio) to obtain the compound Crystallization, chemical properties and spectral data (see Table 1) were identified as a lignan. Its structure is:
[0030]
Embodiment 3
[0031] Example 3: Inhibitory activity of antidiarrheal lignan A on two bacterial strains
[0032] Refer to the US Clinical Laboratory Standardization Committee CLSI (formerly NCCLS) drug susceptibility test protocol to determine the in vitro antibacterial activity of the compound antidiarrheal lignan A.
[0033] Instruments and materials:
[0034] Instrument and reagent analytical balance; 721-2000 type spectrophotometer (Shangdong Gaomi Rainbow Analytical Instrument Co., Ltd.); ZHJH-C luxury ultra-clean workbench (Shanghai Zhicheng Analytical Instrument Manufacturing Co., Ltd.); LDZX vertical pressure steam Sterilizer (Shanghai Shen'an Medical Equipment Factory); WMK-02 electric heating constant temperature incubator (Hubei Huangshi Medical Equipment Factory); No. 11 MSSA (methicillin-sensitive Staphylococcus aureus); No. 27 MRSA (resistant Methicillin and vancomycin-resistant Staphylococcus aureus); MH medium (Beijing Obosing Biotechnology Co., Ltd.); BHI broth (Beijing Obosing Bi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com