Iron-based catalyst for preparation of low carbon olefin from synthetic gas, and preparation method and application thereof
A technology of iron-based catalysts and low-carbon olefins, applied in physical/chemical process catalysts, metal/metal oxides/metal hydroxide catalysts, and hydrocarbon production from carbon oxides, etc., can solve the problem of wide catalyst particle size distribution, hydrocarbon Wide product distribution, high preparation cost and other problems, to achieve the effect of narrow size distribution, uniform particle space distribution and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
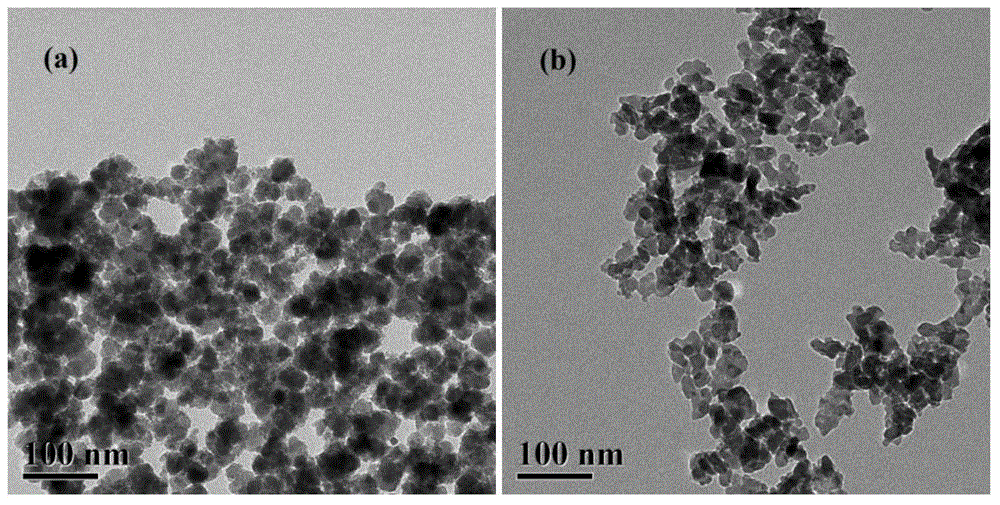
[0042] 31.62gFeCl 3 ·6H 2 O, 12.54 g FeCl 2 4H 2 O and 2.046gNa 2 SiO 3 9H 2 O by Fe 3+ :Fe 2+ :Si=65:35:4 molar ratio mixed into a salt solution, and 5.10mL of 12.1mol / L HCl solution was added. Add 360ml of 1.5mol / L NaOH solution at a constant speed at 60°C under stirring conditions. Within about 2 hours, the pH of the solution was adjusted from acidic to about 10.0. After the dropwise addition, keep the temperature and continue stirring for 1 h, and finally cool to room temperature. After the reaction, use a magnetic field to separate the deposition product, wash it thoroughly with deionized water, and then dry it at 60°C to obtain the catalyst Fe 3 o 4 / SiO 2 Nanocomposite samples, the samples are prepared after grinding, tableting and sieving (20-40 mesh). Fe 3 o 4 The synthesis reaction equation of is:
[0043] Fe 2+ +2Fe 3+ +8OH–→Fe(OH) 2 +2Fe(OH) 3 → Fe 3 o 4 +4H 2 O.
[0044] Reduction conditions: under normal pressure, pure H 2 , the temperatu...
Embodiment 2
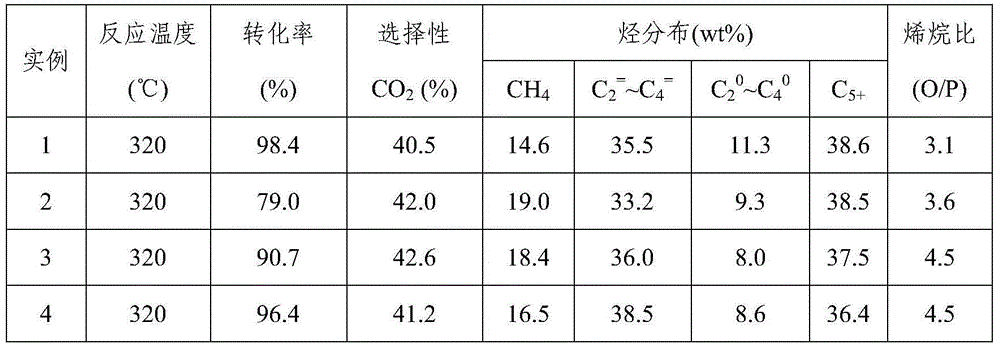
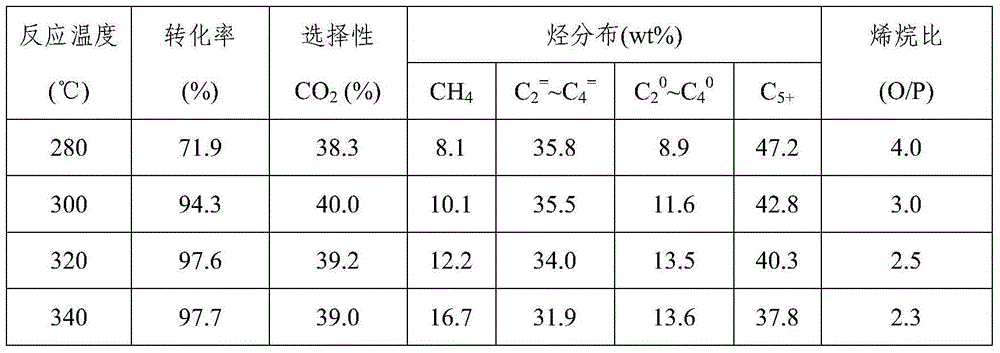
[0046] Take by weighing 4.0g Fe produced by the method of embodiment 1 3 o 4 / SiO 2 Nanocomposite sample, weigh 0.254g KNO 3 , dubbed KNO 3 solution, the above Fe 3 o 4 / SiO 2 Equal volume of the sample was immersed in the above KNO 3 In the solution, stir and stand for 12 hours, dry at 120°C, roast at 400°C for 3 hours, and finally grind, tablet and sieve (20-40 mesh). Reduction conditions: under normal pressure, pure H 2 , the temperature is 350°C, and the space velocity is 1500ml / (h·g cat ), the reduction time is 12h. The reaction conditions are: H 2 / CO=1.0, temperature is 320°C, pressure is 2.0MPa, space velocity is 2000ml / (h g cat ). The results are listed in Table 1.
Embodiment 3
[0048] Take by weighing 4.0g Fe produced by the method of embodiment 1 3 o 4 / SiO 2 Nanocomposite sample, weigh KNO 3 0.254g, measure Mn(NO 3 ) 2 Solution 0.175ml, made into a mixed solution, the above-mentioned Fe 3 o 4 / SiO 2 Immerse equal volume of the sample in the above mixed solution, stir, let it stand for 12 hours, dry at 120°C, and roast at 400°C for 3 hours. The dried product is ground, pressed and sieved (20-40 mesh). Reduction conditions: under normal pressure, pure H 2 , the temperature is 350°C, and the space velocity is 1500ml / (h·g cat ), the reduction time is 12h. The reaction conditions are: H 2 / CO=1.0, temperature is 320°C, pressure is 2.0MPa, space velocity is 2000ml / (h g cat ). The results are listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com