A Synthetic Method of Highly Optically Active Chiral Marine Natural Products
A technology of optical activity and synthetic method, which is applied in the field of synthesis of chiral marine natural products, can solve the problems of complex process, cumbersome route steps, difficult mass production, etc., and achieve wide application prospects, simple synthetic steps, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
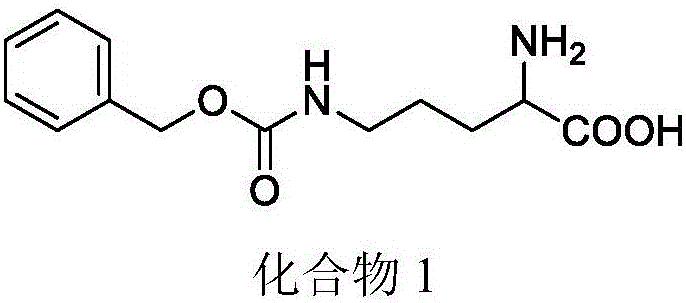
[0055] Example 1: Preparation of 2-amino-5-(((benzyloxy)carbonyl)amino)pentanoic acid
[0056] Dissolve 7.5g of L-ornithine hydrochloride in 89ml of 0.5mol / L sodium hydroxide solution, add 5.55g of anhydrous copper sulfate, react for 15min, then add 6.15g of anhydrous potassium carbonate and 8.2ml of benzyl chloroformate in turn , reacted overnight, filtered and washed to obtain a blue solid, then added saturated EDTA disodium solution, heated to reflux for 2 h, then at room temperature overnight, filtered, washed and dried to obtain a white solid with a yield of 83%.
Embodiment 2
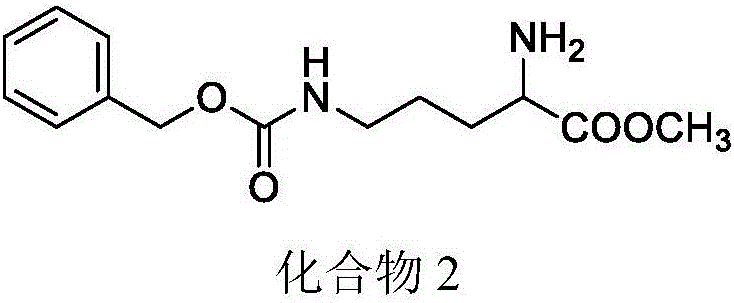
[0057] Example 2: Preparation of methyl 2-amino-5-(((benzyloxy)carbonyl)amino)valerate
[0058] 8.5 ml of acetyl chloride was added dropwise to methanol under ice bath conditions, and the reaction was carried out at room temperature for 10 min. 10.6 g of compound 1 was added to the reaction solution, refluxed for 5 hours, and then the solvent was evaporated, ethyl acetate was added, and after washing and drying, the solvent was evaporated to obtain a light yellow solid with a yield of 85%. 1 H-NMR (300MHz, CDCl 3 ):δ1.36-1.61(m,2H),1.74-1.82(m,2H),2.97-3.03(q,2H),3.74(s,3H),4.05(s,1H),δ5.01(s ,2H),δ7.28-7.40(m,6H),δ8.51(s,3H); 13 C-NMR (100MHz, CDCl 3 ):δ25.3,27.4,40.1,52.9,53.3,66.5,128.0,128.5,128.6,136.7,156.8,170.2.
Embodiment 3
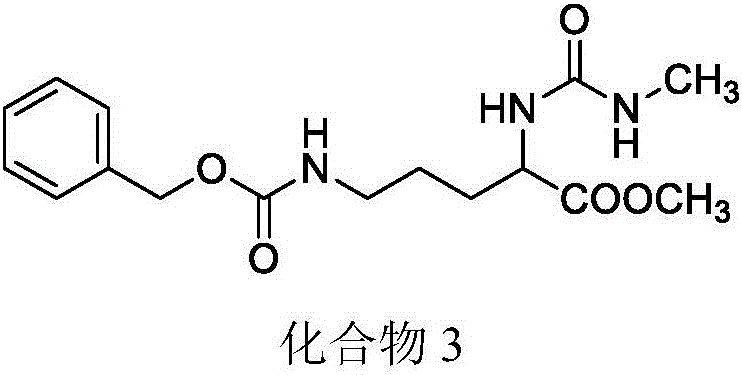
[0059] Example 3: Preparation of methyl 5-(((benzyloxy)carbonyl)amino)-2-(3-methylureido)pentanoate
[0060] Under ice bath conditions, 11.2 g of compound 2 was dissolved in a mixed solution of dichloromethane and saturated sodium bicarbonate, and 5.29 g of triphosgene was added, reacted for 15 min, and then rapidly extracted. The organic phase was dried to obtain a dichloromethane solution of isocyanate. In an ice bath, the dichloromethane solution of isocyanate was dropped into the dichloromethane solution containing methylamine and triethylamine, and the reaction was carried out at room temperature for 1 hour after the drop was completed. After the solvent was evaporated, ethyl acetate was added, the organic phase was washed and dried, and then the solvent was evaporated. Recrystallization gave a white solid with a yield of 78% and a melting point of 114-117°C. 1 H-NMR (300MHz, DMSO-d 6 ):δ1.38-1.49(m,2H),δ1.52-1.69(m,2H),δ2.51-2.54(d,3H),2.95-3.01(q,2H),δ3.60(s, 3H),δ4....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com