Polypeptide and trypsin tablet composition and application thereof
A composition and drug technology, applied in the field of preparation of the above-mentioned chemical products, can solve problems such as changing the properties of active proteins, increasing potential safety hazards, reducing protein activity, etc., achieving the effects of convenient equipment, easy promotion and application, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 polypeptide of the present invention
[0025] After long-term research, the inventors have developed the polypeptide of the present invention and its optimized expression gene sequence in yeast, wherein the nucleotide of the gene is shown in SEQ ID NO: 1, and the amino acid sequence of the polypeptide of the present invention encoded by it is as follows Shown in SEQ ID NO: 2. We entrust Xi'an Xintong Pharmaceutical Research Co., Ltd. to synthesize the gene and clone it into yeast cells. The amplification product was double digested with EcoRI and XbaI and connected between the multiple cloning sites of EcoRI and XbaI of pPICZαA (available from Invitrogen). After being confirmed by sequencing, the positive plasmid was transformed into yeast GS115 strain (available from Invitrogen Company), positive yeast cells were screened out with Zeocin.
[0026] The constructed positive yeast cells were inoculated in 25ml seed medium, and cultured wi...
Embodiment 2
[0028] Embodiment 2 Stability study of the polypeptide of the present invention
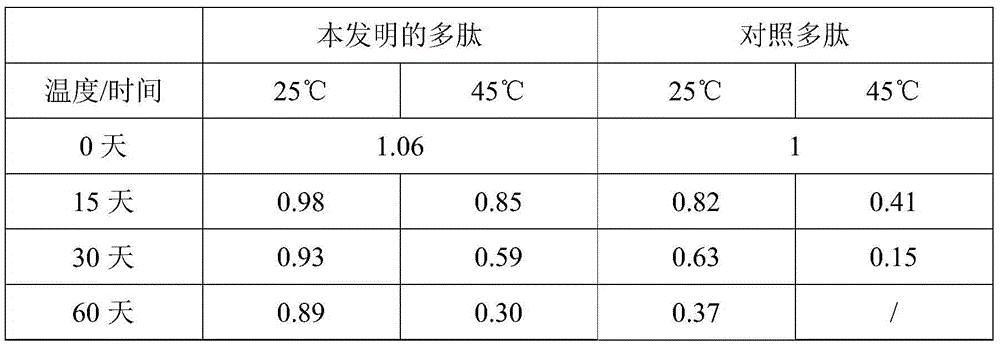
[0029] Referring to the protease activity detection method described in Harrach et al. (JProteinChem, 14:41-52), respectively detect the effect of the polypeptide of the present invention prepared in Example 1 and the reference polypeptide prepared in high temperature storage for different times on the substrate L-Pyr-Phe-Leu- Hydrolytic activity of pNA. For the convenience of comparison, the activity of 1 mg of the control polypeptide stored at high temperature for 0 days (that is, taken out from the storage state at -20°C for detection immediately) was counted as 1, and the relative activities of 1 mg of different polypeptides stored under different conditions are shown in Table 1. shown.
[0030] The stability of table 1 polypeptide
[0031]
[0032] As can be seen from Table 1, the activity of an equivalent amount of polypeptide of the present invention and the wild-type control polypept...
Embodiment 3 2
[0033] The preparation of embodiment 3 two enzyme tablets
[0034] Take 502 grams of starch and 130 grams of lactose, add 50% (V / V) ethanol to make a soft material, granulate, dry at 45°C until the water content is less than 5% (W / W), pass through a 20-mesh sieve, That is, the ball core is obtained.
[0035] Get 90 grams of the polypeptide of the present invention prepared in Example 1, 48 grams of trypsin and 100 grams of rutin, mix evenly with 5 grams of silicon dioxide micropowder, 25 grams of microcrystalline cellulose and the above-mentioned ball core, press into tablets, and press into 1000 piece.
[0036] Get 160 grams of oprdry standard coating powder, dissolve in 4L of 75% (V / V) ethanol, and prepare enteric coating solution. The above-mentioned formed tablets are placed in the fluidized bed, start the fluidized bed, and spray the enteric coating solution to these formed tablets with atomizing nozzles, wherein the parameters of the fluidized bed setting are: bed temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com