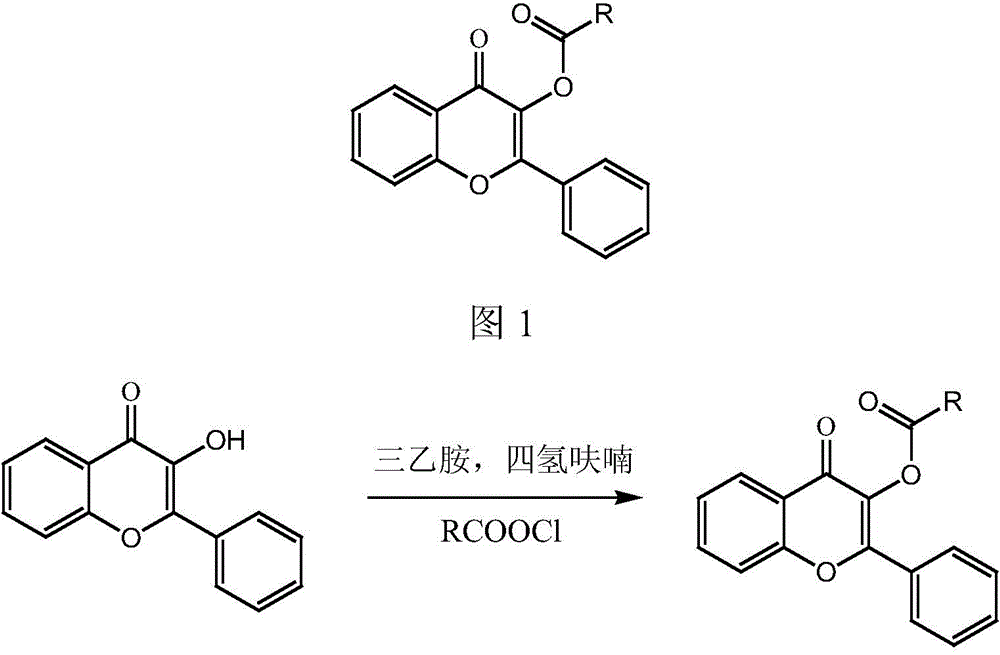
High-specificity fluorescent probe of human carboxylesterase CES2 and application thereof
A high-specificity, fluorescent probe technology, applied in the field of high-specificity fluorescent probes, to achieve the effect of simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0044] The chemical synthesis of embodiment 13-hydroxyflavone 4-ethylbenzoyl ester
[0045] (1) Slowly add 0.6 mmol of p-ethylbenzoyl chloride (dissolved in 5 mL of tetrahydrofuran) dropwise to 10 mL of tetrahydrofuran solution containing 0.5 mmol of 3-hydroxyflavone and 0.625 mmol of triethylamine, and control the temperature at 0°C ;
[0046] (2) After stirring in an ice bath for 1 hour, the solution was brought to room temperature and stirred overnight;
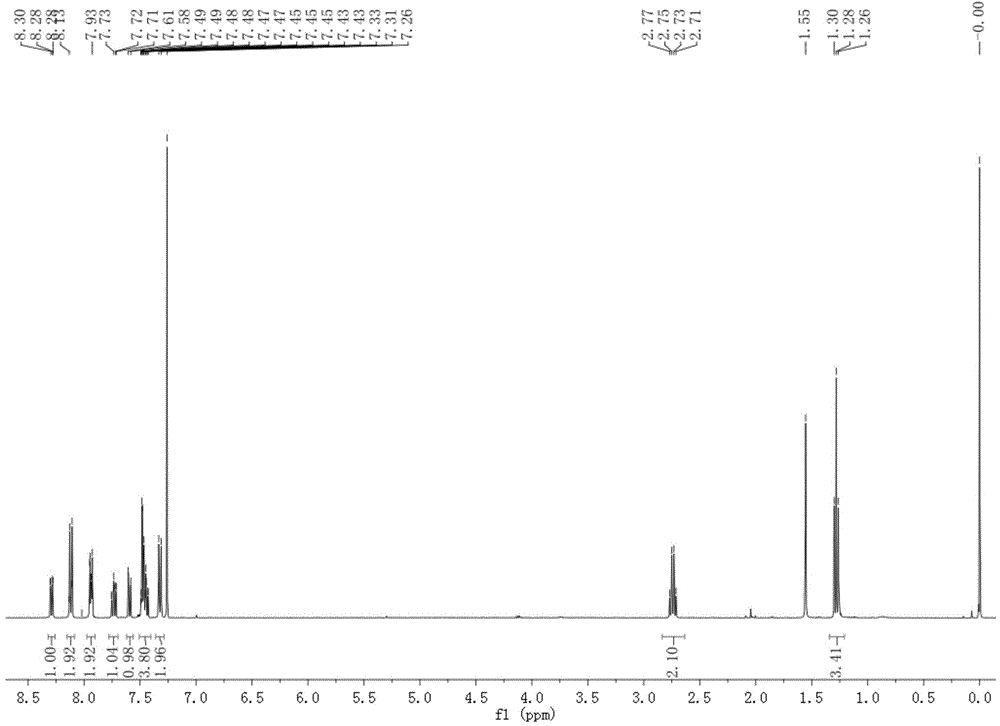
[0047] (3) The solvent was removed from the reaction solution under reduced pressure, and the residual solid was purified by silica gel chromatography, eluting with ethyl acetate-n-hexane (1:3 v / v), to obtain 155 mg of pure white solid powder. δ( 1 H-NMR, 400MHz, CDCl 3 ):8.29(1H,dd),8.15-8.09(2H,m),7.98-7.90(2H,m),7.73(1H,ddd),7.59(1H,dd),7.50-7.42(4H,m), 7.32(2H,d), 2.74(2H,q), 1.35-1.22(3H,m). (See the synthetic route diagram figure 2 ) (see the hydrogen spectrum image 3 ).
Embodiment 23
[0048] The chemical synthesis of embodiment 23-hydroxyflavone 4-chlorobenzoyl ester
[0049] (1) Slowly add 0.6 mmol of p-chlorobenzoyl chloride (dissolved in 5 mL of tetrahydrofuran) dropwise to 10 mL of tetrahydrofuran solution containing 0.5 mmol of 3-hydroxyflavone and 0.625 mmol of triethylamine, and control the temperature at 0°C;
[0050] (2) After stirring in an ice bath for 1 hour, the solution was brought to room temperature and stirred overnight;
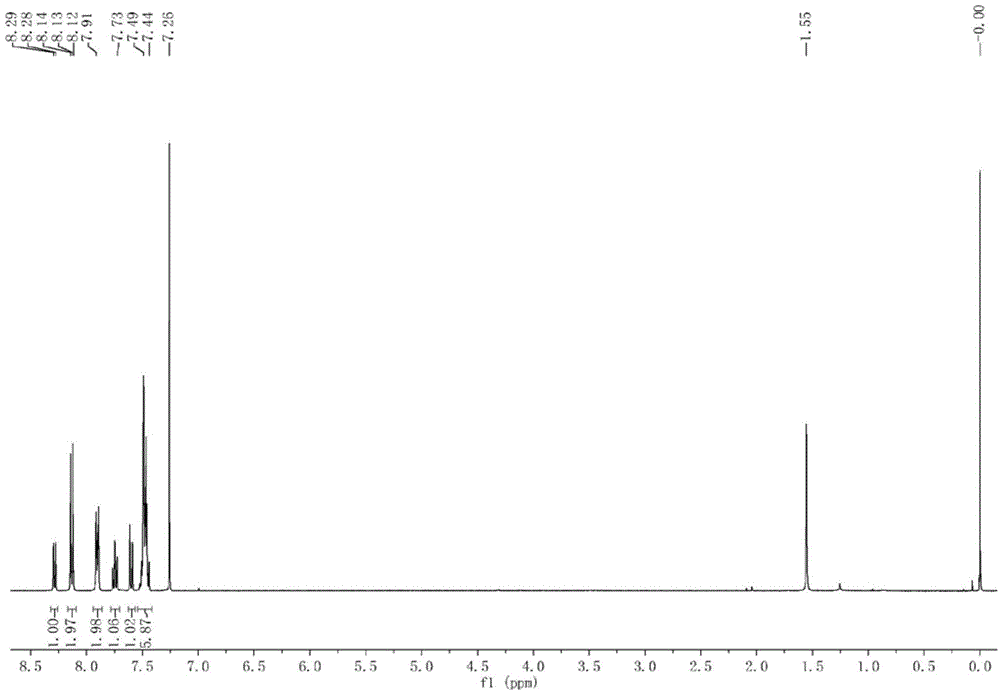
[0051] (3) The solvent was removed from the reaction solution under reduced pressure, and the residual solid was purified by silica gel chromatography, eluting with ethyl acetate-n-hexane (1:3 v / v), to obtain 152 mg of pure white solid powder. δ( 1 H-NMR, 400MHz, CDCl 3 ):8.29(1H,dd),8.13(2H,m),7.92-.89(2H,m),7.77-7.72(1H,ddd),7.60(1H,dd),7.49-7.46(6H,m) . (Synthetic route map see figure 2 ) (see the hydrogen spectrum Figure 4 ).
Embodiment 33
[0052] The chemical synthesis of embodiment 33-hydroxyflavone 4-methylbenzoyl ester
[0053] (1) Slowly add 0.6 mmol of p-toluoyl chloride (dissolved in 5 mL of tetrahydrofuran) dropwise to 10 mL of tetrahydrofuran solution containing 0.5 mmol of 3-hydroxyflavone and 0.625 mmol of triethylamine, and control the temperature at 0°C ;
[0054] (2) After stirring in an ice bath for 1 hour, the solution was brought to room temperature and stirred overnight;
[0055] (3) The solvent was removed from the reaction solution under reduced pressure, and the residual solid was purified by silica gel chromatography, eluting with ethyl acetate-n-hexane (1:3 v / v), to obtain 151 mg of pure white solid powder. δ( 1 H-NMR, 400MHz, CDCl 3 ):8.30(1H,dd),8.10-8.12(2H,m),7.96-7.94(2H,m),7.75(1H,ddd),7.61(1H,dd),7.50-7.44(4H,m), 7.31(2H,d), 2.46(2H,s). (See the synthetic route diagram figure 2 ) (see the hydrogen spectrum Figure 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com