Pure organic D-pi-A type photosensitive dye as well as preparation method and application thereof
A photosensitive dye, organic technology, applied in the field of pure organic D-π-A photosensitive dye and its preparation, to achieve the effect of convenient structure modification, simple synthesis route and reliable technical support
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
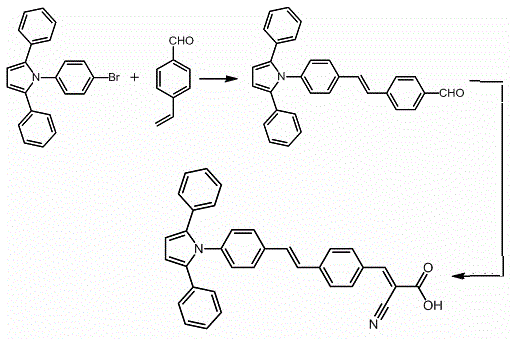
[0029] Embodiment 1, the synthetic method of triphenylpyrrole D-π-A type dye, see figure 1 .
[0030] Under the protection of argon, add 11.2 mg palladium acetate (0.05 mmol), 0.132 g p-formylstyrene (1.0 mmol), 0.374 g 1-(p-bromophenyl)-2,5-diphenyl pyrrole (1.0 mmol), 0.322 g tetrabutylammonium bromide (1.0 mmol), 60.4 mg tris(o-methylphenyl)phosphine (0.2 mmol), 8 mL N,N'-dimethylformamide and 5 mL of triethylamine, heated to 90 °C, maintained at this temperature and stirred for 24 hours. After cooling to room temperature, the solid was filtered off, and the solvent and triethylamine were distilled off under reduced pressure to obtain an intermediate product. Disperse the intermediate in 10 mL of anhydrous CH 3 CN, add 0.34 g cyanoacetic acid (4.0 mmol) and 0.1 mL piperidine, heat to 80 ° C and reflux for 3 hours. After cooling to room temperature, the solvent was removed by vacuum distillation. The residue was purified by column chromatography (the eluent was dichloro...
Embodiment 2
[0032] Example 2, the application of triphenylpyrrole D-π-A type photosensitizing dye as a dye sensitizer for preparing solar cells
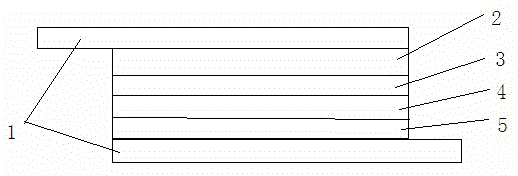
[0033] The preparation method of the photoanode sensitized by triphenylpyrrole D-π-A type photosensitizing dye: see Figure 5 . FTO glass sheet 1 was purchased from Dalian Qiseguang Technology Co., Ltd., and the surface resistance of FTO glass 1 was 15 Ω · cm –2. Before use, FTO glass slide 1 was sonicated in a detergent solution for 30 min, washed with water and ethanol, and then soaked in 40 mM TiCl 4 Soak in aqueous solution for 30 minutes at 70°C, then wash with water and ethanol. TiO was prepared by screen printing 6 times 2 Nanocrystalline Photoelectrode 2. TiO 2 The slurry was made from commercial P25 TiO 2 Nanoparticles, ethyl cellulose (as a binder) and α-terpineol (as a solvent). TiO 2 Sintered at 450° C. for 1 hour after screen printing onto FTO glass sheet 1 . 2.0×2.0cm 2 TiO 2 The thickness of the film is 12 μm. After ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com