Application of chlorogenic acid in preparation of medicines for treating parkinson disease
A technology for Parkinson's disease and chlorogenic acid is applied in the field of use of chlorogenic acid in the preparation of a drug for treating Parkinson's disease, and can solve the problems of striatal DA deficiency, basal ganglia neuromodulation disorder and the like, and achieve a pathological state Effects of improving and treating, inhibiting substantia nigra apoptosis and reducing oxidative stress damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1. In vivo pharmacodynamic investigation of chlorogenic acid on Parkinson's model mice
[0042] Experimental Materials
[0043] 1.1 Experimental reagents and instruments
[0044] Chlorogenic acid (Sichuan Jiuzhang Biotechnology Co., Ltd.), rotenone (SIGMA), dimethyl sulfoxide (DMSO, SIGMA), corn oil (Shandong Luhua Group Co., Ltd.), mouse α-SYN monoclonal antibody, SABC immune tissue Chemical kits were purchased (Wuhan Boster Company), mouse tyrosine hydroxylase monoclonal antibody, rabbit microtubule-associated protein light chain 3 polyclonal antibody (Biyuntian), malondialdehyde content and superoxide dismutase, gluten Glutathione peroxidase and catalase kit (Nanjing Jiancheng Biological Engineering Co., Ltd.). The rest of the reagents were of domestic commercially available analytical grade.
[0045] 1.2 Animal modeling and grouping
[0046] In this experiment, a mouse model of Parkinson's disease was established by subcutaneous injection of rotenone. T...
Embodiment 2
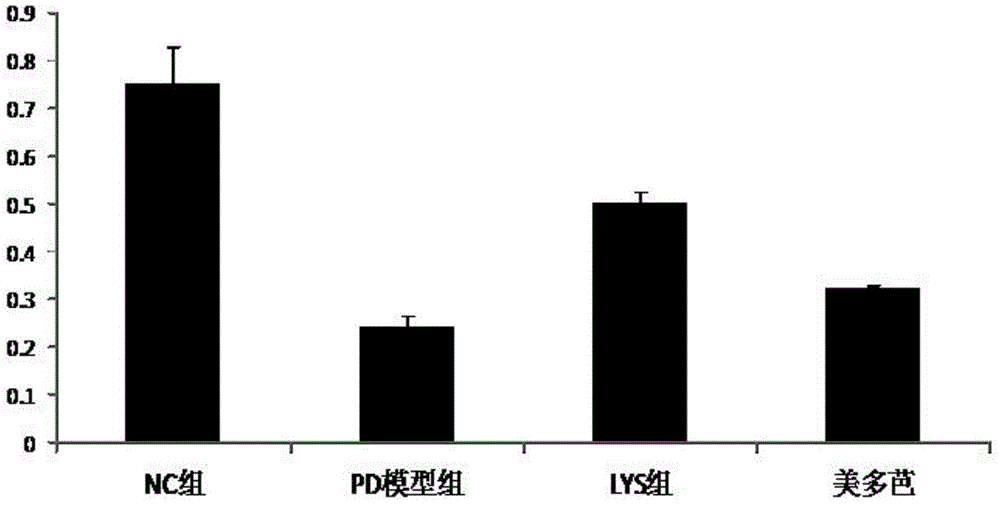
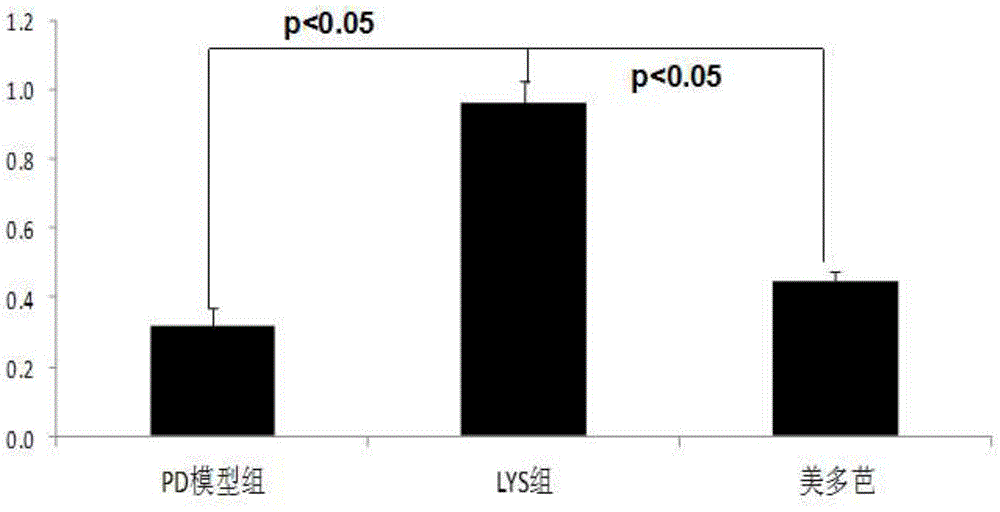
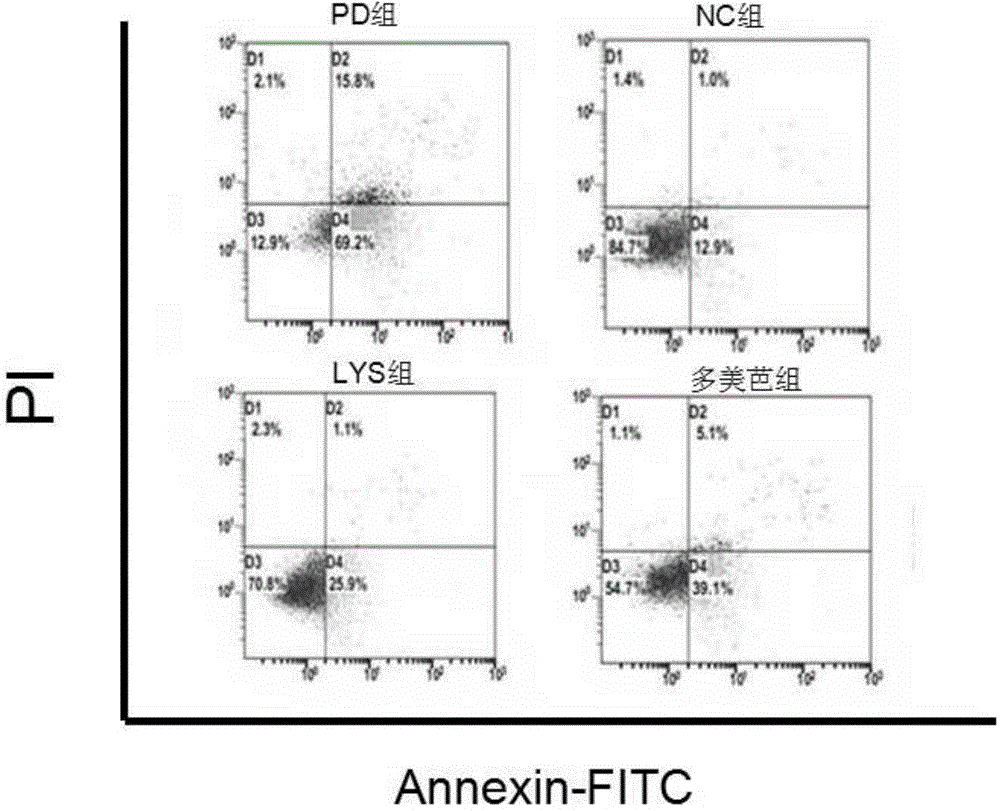
[0084] Example 2. Investigation of the effects of chlorogenic acid on the HMGCS2 gene and the corresponding trihydroxytrimethyl-CoA synthetase 2 activity, ketone body content in the body and the apoptosis of substantia nigra cells in Parkinson's model mice
[0085] 1. Experimental materials
[0086] 1.1 Experimental reagents and instruments
[0087] Chlorogenic acid, Madopar (dopasrazine tablets, Shanghai Roche Pharmaceutical Co., Ltd.), microplate reader (Rebo mk3 microplate reader from Finland), real-time fluorescent quantitative PCR instrument (Applied Biosystems, Inc.; ABI), total RNA Extraction Kit (Tiangen Biochemical Technology Co., Ltd.), TIANScript cDNA First Strand Synthesis Kit (Tiangen Biochemical Technology Co., Ltd.), Annexin-FITC-PI Double Staining Apoptosis Detection Kit (Biyuntian), Mouse Ketone Body Detect ELISA kit (Sigma), flow cytometer (Beckman), etc.
[0088] 1.2 Animal model and grouping
[0089] Of 30 BALB / C mice, 10 were randomly selected as the no...
Embodiment 3
[0128] Embodiment 3: Prepare freeze-dried powder injection with chlorogenic acid
[0129] 1. Extraction of chlorogenic acid:
[0130] The chlorogenic acid crude drug used in this example is extracted and purified from Flos Lonicerae, with a purity of 99.52%.
[0131] 2. Preparation of chlorogenic acid freeze-dried powder injection
[0132] 2.1 Prescription:
[0133]
[0134] Completely dissolve the above prescription in water for injection, filter, and fine filter with a 0.22 μm sterilizing microporous membrane, adjust the pH, and make a total of 1000 2ml powder injections according to the routine operation of sterile powder injections, each Contains chlorogenic acid 40mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com