Method for recovering nickel, cobalt, iron and silicon from laterite-nickel ore through united leaching technology
A laterite nickel ore, combined leaching technology, applied in the field of recovering nickel, cobalt, iron and silicon, can solve the problems of low nickel and cobalt leaching rate, high acid consumption, poor economic benefits, etc., and achieve high nickel and cobalt recovery rate, The effect of low acid consumption and fast recovery speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
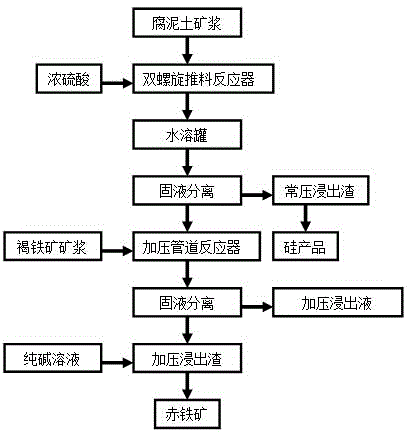
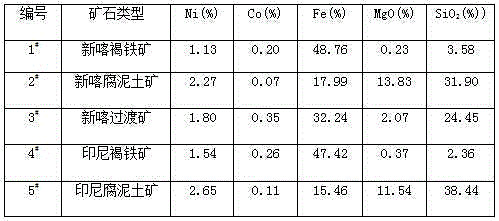
[0033] Take 500Kg 2 # Add 500Kg of water to saprolite ore (dry) to make saprolite slurry, prepare 500Kg of concentrated sulfuric acid with a mass fraction of 98%, heat the saprolite slurry to 60°C, and the concentrated sulfuric acid to 200°C, then use a mortar pump and a concentrated sulfuric acid pump to The heated saprolite slurry and concentrated sulfuric acid are simultaneously fed into the feed port of the twin-screw pusher reactor. After rapid mixing, the saprolite slurry and concentrated sulfuric acid are forced to flow into the double-screw pusher reactor for rapid reaction to dissolve soluble non-ferrous metals. and soluble iron, after reacting for 1 minute, push the reaction material out of the double-screw pusher reactor. Cool down to below 60°C, simply crush the reaction material of the loose honeycomb solid paste and pour it into a water immersion tank, add 1500Kg of water, stir for 30 minutes to dissolve in water, and pump the resulting slurry into a plate and fr...
Embodiment 2
[0047] Take 500Kg 5 # Add 600Kg of water to saprolite ore (dry) to make saprolite slurry, prepare 500Kg of concentrated sulfuric acid with a mass fraction of 98%, heat the saprolite slurry to 100°C, and the concentrated sulfuric acid to 150°C, then use a mortar pump and a concentrated sulfuric acid pump to The heated saprolite slurry and concentrated sulfuric acid are simultaneously fed into the feed port of the twin-screw pusher reactor. After rapid mixing, the saprolite slurry and concentrated sulfuric acid are forced to flow into the double-screw pusher reactor for rapid reaction to dissolve soluble non-ferrous metals. And soluble iron, after reacting for 12 minutes, push out the reaction material into the twin-screw pusher reactor. Cool down to below 60°C, simply crush the reaction material of the loose honeycomb solid paste and pour it into a water immersion tank, add 1920Kg of water, stir for 30 minutes to dissolve in water, and pump the resulting slurry into a plate and...
Embodiment 3
[0061] The normal-pressure acid leaching stage of the present embodiment is the same as that of Example 1, and in the pressure leaching stage, 1 # Sinka Limonite changed to 4 # Indonesian limonite.
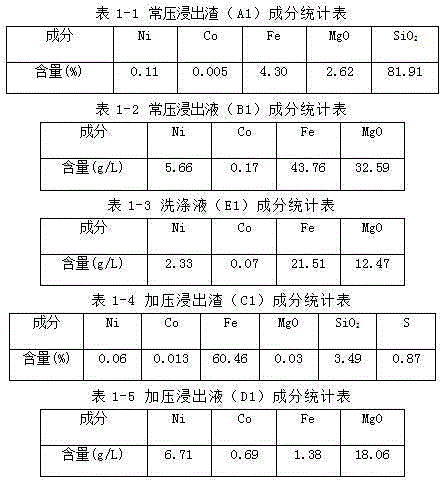
[0062] Take 30Kg4 # Limonite (dry), add 60L of washing liquid (E1) to make limonite slurry, heat the limonite slurry to 95°C, add it to the circulation tank of the pressurized pipeline reactor, and then add it to the pressurized pipeline reactor Add normal pressure leaching solution (B1) heated to 95°C into the circulation tank to make the final pH value of the reaction material 1.0. After sealing the circulation tank, turn on the booster pump, and at the same time turn on the heat conduction oil heating device of the pressurized pipeline reactor to control the temperature and heat. After pressure leaching for 60 minutes at a pressure of 2.0 MPa and a temperature of 223°C, the Fe in the atmospheric pressure leaching solution (B1) 3+ Hydrolyze into hematite precipitation and rel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com