Method for preparing reverse osmosis composite membrane containing azobenzene polyamide
A technology of reverse osmosis composite membrane and nitrogen-benzene polyamide, applied in semi-permeable membrane separation, chemical instruments and methods, membrane technology, etc., to achieve strong anti-pollution performance, high desalination rate and water flux, and good mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
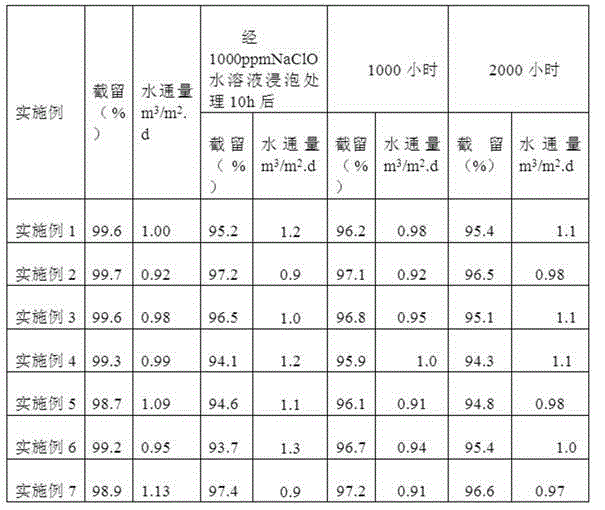
Examples
Embodiment 1
[0041] A production method of azobenzene-containing polyamide reverse osmosis composite membrane, comprising the following steps:
[0042] (1) Synthesis of p-aminoazobenzene derivatives (A)
[0043] Take 5.5g (0.04mol) p-nitroaniline, add 16g (0.16mol) concentrated HCl and 20gH 2 0, heated to dissolve under stirring, and then cooled in an ice-water bath to precipitate fine crystals of hydrochloride. Take another 3.3g (0.048mol) sodium nitrite, add 18gH 2 0 to form a solution, quickly added dropwise to the above hydrochloride, and reacted for 30 min under vigorous stirring. Use starch-potassium iodide test paper to check whether the reaction is complete, and use urea to remove excess nitrous acid generated in the reaction. The reaction solution was filtered to obtain a clear yellow filtrate which was the diazonium salt solution of p-nitroaniline.
[0044] Take 8.3g (0.048mol) of p-aminobenzenesulfonic acid and 1.0g (0.01mol) of concentrated hydrochloric acid, add 30g of H2O...
Embodiment 2
[0063] A production method of an azobenzene polyamide reverse osmosis composite membrane, comprising the following steps:
[0064] (1) Synthesis of p-aminoazobenzene derivative (A)
[0065] Substitute 5.5g (0.04mol) of p-nitroaniline in step (1) of Example 1 with 9.2g (0.04mol) of sodium p-aminobenzenesulfonate, and the rest are the same as in Example 1. The resulting product is p-aminoazosulfonic acid benzene (A2).
[0066] (2) Synthesis of side chain azoamide functional monomer (B)
[0067] Substitute 9.7 g (0.04 mol) of product A1 in step (2) of Example 1 with 13.5 g (0.04 mol) of product A2, and the rest are the same as in Example 1. The resulting product is methacrylamide azosulfobenzene (B2).
[0068] (3) Synthesis of azoamide homopolymer (C) with ω-halogen group at the end
[0069] Replace 31.1 g (0.1 mol) of product B1 in step (3) of Example 1 with 40.5 g (0.1 mol) of product B2, and the rest are the same as in Example 1. The resulting product is an azoamide homop...
Embodiment 3
[0073] A production method of an azobenzene polyamide reverse osmosis composite membrane, comprising the following steps:
[0074] (1) Synthesis of p-aminoazobenzene derivative (A)
[0075] In the step (1) of Example 1, p-nitroaniline was replaced with p-aminobenzoic acid, and the rest were the same as in Example 1. The obtained product is p-aminoazocarboxybenzene (A 3 ).
[0076] (2) Synthesis of side chain azoamide functional monomer (B)
[0077] Same as Example 1. The resulting product is methacrylamide azocarboxybenzene (B 3 ).
[0078] (3) Synthesis of azoamide homopolymer (C) containing ω-halogen group at the end group
[0079] Same as Example 1. The resulting product is an azoamide homopolymer containing an ω-halogen group at the end (C 3 ).
[0080] (4) Synthesis of Azobenzene & MMA Block Copolymer (D)
[0081] Same as Example 1.
[0082] (5) Synthesis of chain-type anionic / cationic azoamide homopolymer (E)
[0083] 40g of phenol in Example 1 was replaced w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com