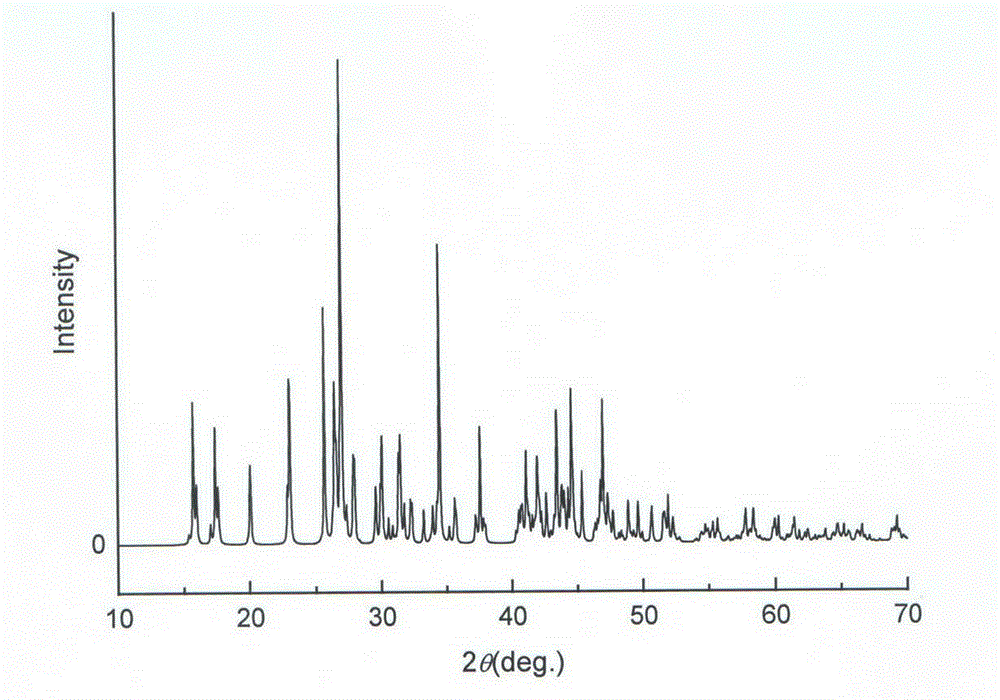
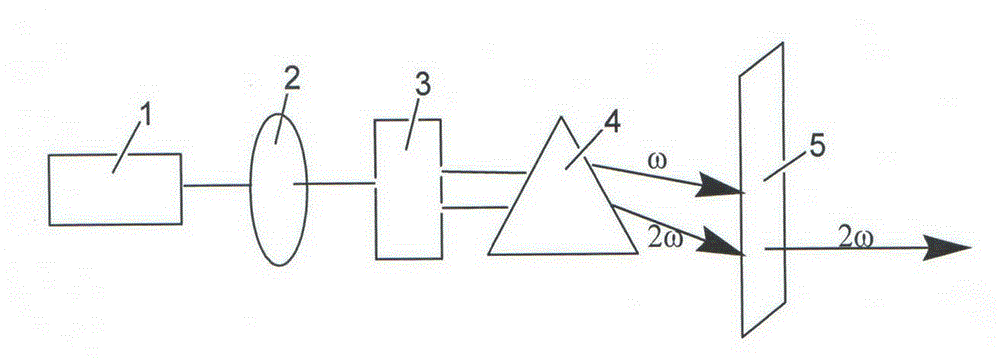
Compound mono-boric dihydroxyl strontium decaborate monohydrate nonlinear optical crystal and preparation method and use thereof
A boric acid dihydroxystrontium decaborate, nonlinear optics technology, applied in nonlinear optics, chemical instruments and methods, optics and other directions, can solve the problems of difficult crystal growth, weak bonding force, difficult processing, etc., achieves simple preparation, Low cost, fast growth effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] With the chemical reaction formula 2SrCl 2 +11H 3 BO 3 →Sr 2 (B 5 o 8 (OH)) 2 B(OH) 3 ·H 2 O+4Cl - +11H 2 O+4H + To prepare crystals, the specific steps are as follows:
[0044] a. Mole ratio of SrCl 2 :H 3 BO 3 =1:4, the SrCl 2 Add to the polytetrafluoroethylene liner of a 23mL autoclave, add H 3 BO 3 , and then add 10 mL of deionized water to make it fully mixed to obtain a mixed solution;
[0045] b. Add 1 mL of LiOH solution with a mineralizer concentration of 3 mol / L to the mixed solution in step a and mix;
[0046] c. Tighten the polytetrafluoroethylene-lined lid with the mixed solution in step b, put it into a corresponding clean and pollution-free autoclave, and tighten the piston of the reactor;
[0047] d. Place the autoclave in step c in an incubator, raise the temperature to 180°C at a rate of 20°C / h, keep the temperature constant for 3 days, and then lower it to room temperature at a rate of 2°C / h;
[0048] e. Open the high-pressure react...
Embodiment 2
[0050] According to the chemical reaction formula 2Sr(CH 3 COO) 2 +11H 3 BO 3 →Sr 2 (B 5 o 8 (OH)) 2 B(OH) 3 ·H 2 O+4CH 3 COO - +9H 2 O+4H + Prepare crystals:
[0051] a. Sr(CH in molar ratio) 3 COO) 2 ·H 2 O:H 3 BO 3 =1:2, Sr(CH 3 COO) 2 ·H 2 O was added to the polytetrafluoroethylene liner of an autoclave with a volume of 80 mL, and H 3 BO 3 , and then add 35mL of deionized water to make it fully mixed to obtain a mixed solution;
[0052]b. Add 0.5 mL of NaOH solution with a mineralizer concentration of 3 mol / L to the mixed solution in step a and mix;
[0053] c. Tighten the polytetrafluoroethylene-lined lid with the mixed solution in step b, put it into a corresponding clean and pollution-free autoclave, and tighten the piston of the reactor;
[0054] d. Place the autoclave in step c in an incubator, raise the temperature to 200°C at a rate of 30°C / h, keep the temperature constant for 6 days, and cool to room temperature naturally;
[0055] e. Open ...
Embodiment 3
[0057] With the chemical reaction formula 2SrSO 4 +11H 3 BO 3 →Sr 2 (B 5 o 8 (OH)) 2 B(OH) 3 ·H 2 O+2SO 4 2- +11H 2 O+4H + Prepare crystals:
[0058] a. SrSO in molar ratio 4 :H 3 BO 3 =1:6, the SrSO 4 Add to the polytetrafluoroethylene liner of a 23mL autoclave, add H 3 BO 3 , and then add 10 mL of deionized water to make it fully mixed to obtain a mixed solution;
[0059] b. Add 0.5 mL of KOH solution with a mineralizer concentration of 3 mol / L to the mixed solution in step a and mix;
[0060] c. Tighten the polytetrafluoroethylene-lined lid with the mixed solution in step b, put it into a corresponding clean and pollution-free autoclave, and tighten the piston of the reactor;
[0061] d. Place the autoclave in step c in a thermostat, raise the temperature to 210°C at a rate of 50°C / h, keep the temperature constant for 10 days, and then lower it to room temperature at a rate of 30°C / h;
[0062] e, open the autoclave, filter the solution containing the cry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com