A kind of sustained-release capsule containing ibuprofen racemate or dextroform and preparation process thereof
A technology for sustained-release capsules and a preparation process, which is applied in the field of sustained-release capsules containing ibuprofen, can solve the problems of complex preparation process, unfavorable environmental protection, and unfavorable large-scale production of preparations, and achieves simple preparation process, complete drug release, and improved affinity. water-based effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
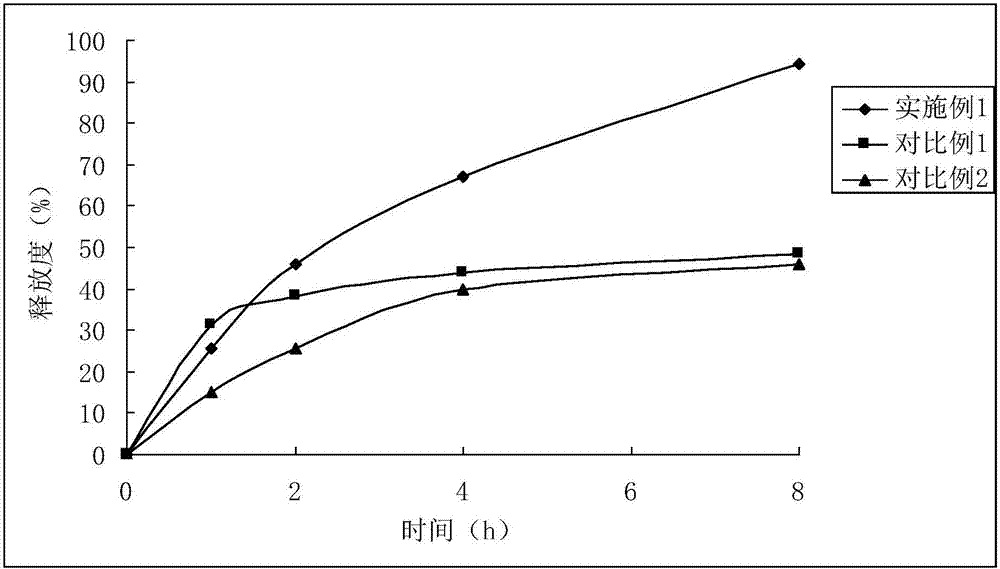
Embodiment 1
[0029] The preparation of embodiment 1 ibuprofen sustained-release capsules
[0030] (1) Content prescription
[0031] Ibuprofen 300g
[0032] Lactose 100g
[0033] 1% sodium lauryl sulfate aqueous solution appropriate amount
[0034] (2) Slow-release coating layer
[0035]
[0036] Preparation Process:
[0037] (1) The ibuprofen is pulverized through a 100 mesh sieve, and the prescription amount is weighed to mix the ibuprofen and sieved lactose evenly, adding a 1% sodium lauryl sulfate aqueous solution to granulate, and passing through a 20 mesh sieve , dried at 40°C, passed through a 20-mesh sieve and granulated to obtain the contents of the capsule;
[0038] (2) filling the capsule contents prepared in step (1) into the capsule shell to obtain ibuprofen immediate-release capsules;
[0039] (3) Weigh ethyl cellulose and hydroxypropyl cellulose and add them to ethanol and stir until completely dissolved, add magnesium stearate and stir evenly to obtain a slow-releas...
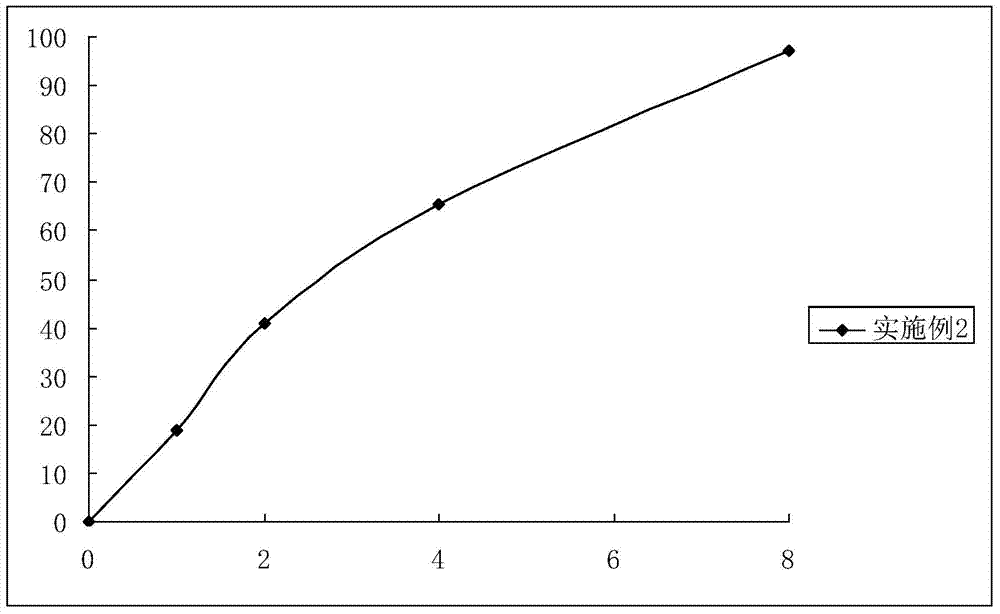
Embodiment 2
[0041] The preparation of embodiment 2 ibuprofen sustained-release capsules
[0042] (1) Content prescription
[0043] Ibuprofen 300g
[0044] Mannitol 100g
[0045] 1% Tween 80 aqueous solution appropriate amount
[0046] (2) Slow-release coating layer
[0047]
[0048] Preparation Process:
[0049] (1) Ibuprofen is pulverized through a 100 mesh sieve, and the prescription amount is taken by weighing ibuprofen and mannitol that has been sieved and mixed evenly, adding mass volume percentage is 1% Tween 80 aqueous solution to granulate, crossing a 20 mesh sieve, 40 ℃ drying, passing through a 20-mesh sieve for granulation to obtain the contents of the capsule;
[0050] (2) filling the capsule contents prepared in step (1) into the capsule shell to obtain ibuprofen immediate-release capsules;
[0051] (3) Add ethyl cellulose and hydroxypropyl cellulose to ethanol and stir until completely dissolved, add magnesium stearate and stir evenly to obtain a slow-release coating ...
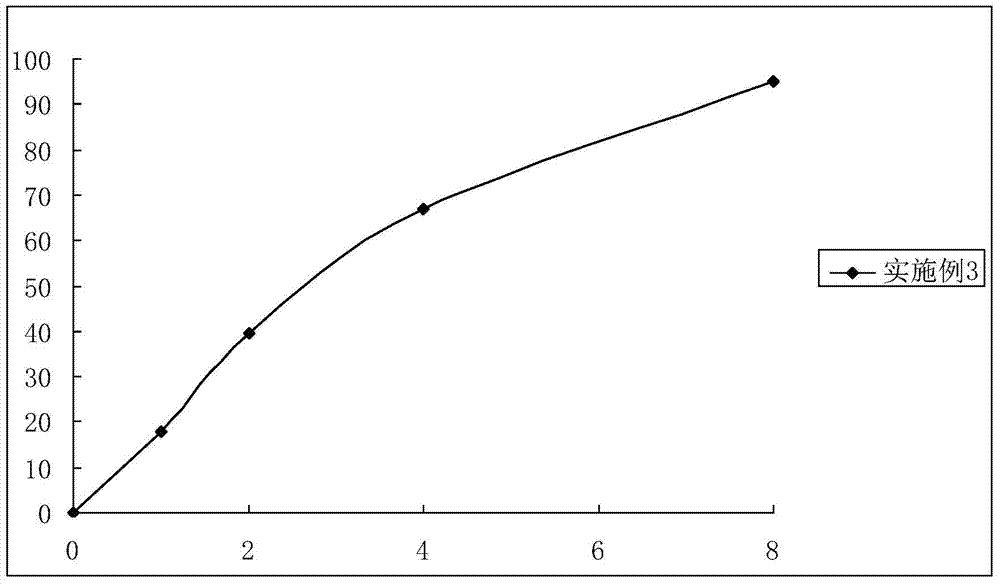
Embodiment 3
[0053] The preparation of embodiment 3 Dexibuprofen sustained-release capsules
[0054] (1) Content prescription
[0055] Dexibuprofen 300g
[0056] Sorbitol 100g
[0057] 1% aqueous solution of poloxamer
[0058] (2) Slow-release coating layer
[0059]
[0060]
[0061] Preparation Process:
[0062] (1) Dexibuprofen is pulverized through a 100-mesh sieve, and the recipe quantity is taken by weighing dextrobuprofen and sorbitol that has been sieved and mixed uniformly, adding a mass volume percentage of 1% poloxamer aqueous solution to granulate, passing 20-mesh sieve, dry at 40°C, pass through a 20-mesh sieve and granulate to obtain the contents of the capsule;
[0063] (2) filling the capsule contents prepared in step (1) into the capsule shell to obtain Dexibuprofen immediate-release capsules;
[0064] (3) Add ethyl cellulose and hydroxypropyl cellulose to ethanol and stir until completely dissolved, add magnesium stearate and stir evenly to obtain a slow-releas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com