A kind of preparation method of urea linkage multifunctional synergistic antioxidant stabilizer
An antioxidant stabilizer, multifunctional technology, applied in the preparation of organic compounds, chemical instruments and methods, preparation of carbamate derivatives, etc., can solve the problems of weakening strength, embrittlement cracking, damage, etc. The effect of improving the use value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
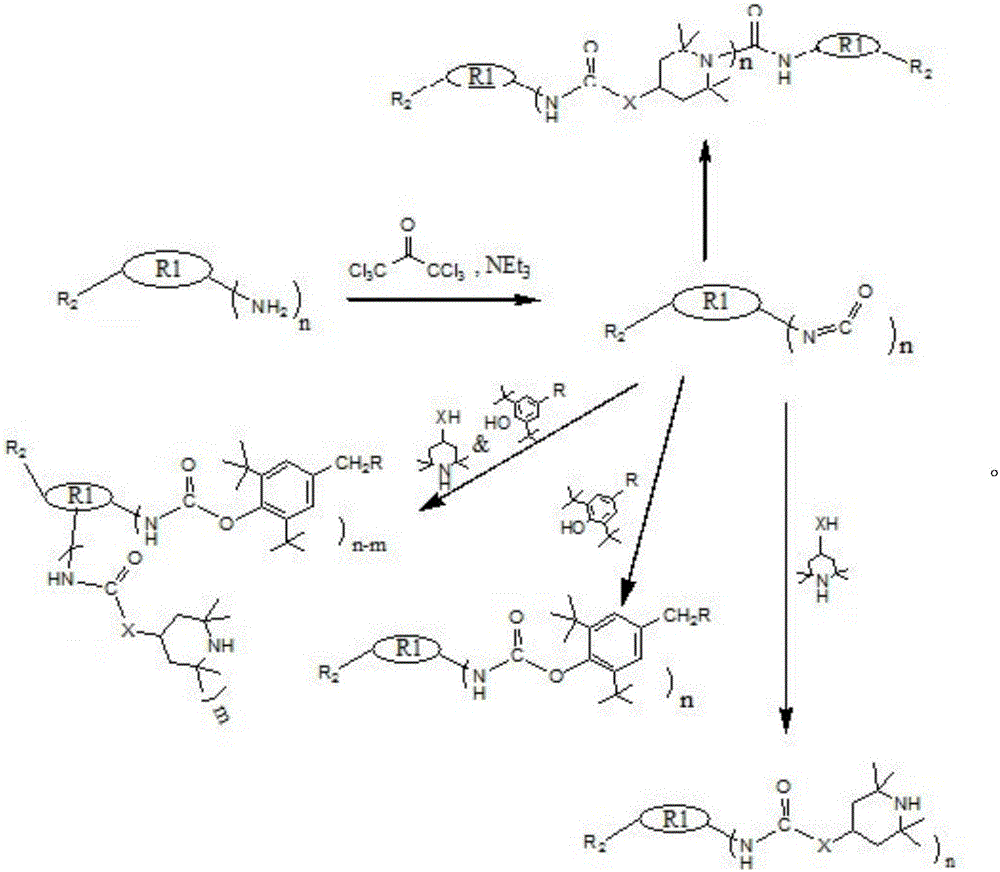
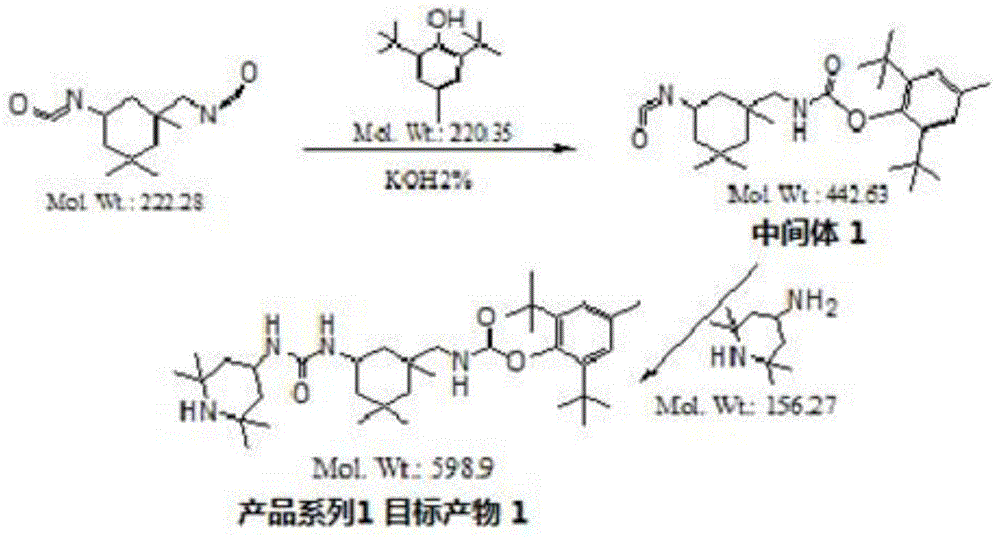
[0036] 1. Synthetic route:
[0037]
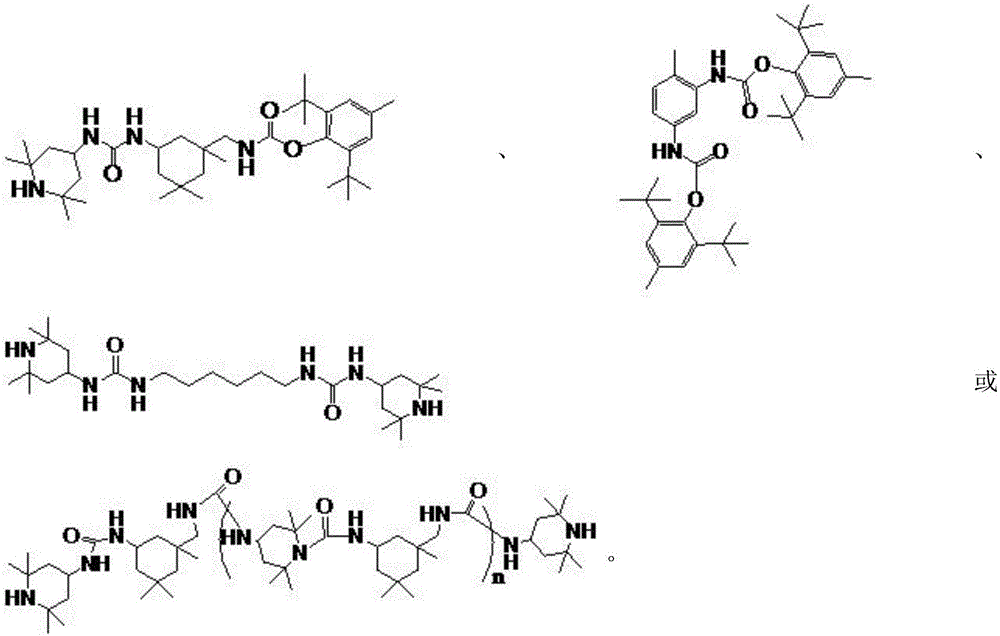
[0038] 2. The chemical structural formula of the product:
[0039]
[0040] 3. Preparation method:
[0041] 2 g of isophorone diisocyanate (8.99 mmol) and 2.18 g of 2,6-di-tert-butyl-p-cresol (9.89 mmol) were dissolved in 20 ml of dry acetone, and 0.03 g of KOH was added under nitrogen protection. The mixture was stirred at room temperature for 30 minutes and heated to 50°C for 18 hours. The reaction was monitored by TLC until the diisocyanate disappeared in the reaction system. The reaction was cooled to room temperature and stirred at room temperature for 3 hours. Remove acetone under vacuum, add EtOAc and saturated NaCl as a solution, extract the product to the organic phase (20mlx3), dry the organic phase with anhydrous Na2SO4, filter, and concentrate the filtrate under vacuum, in a mixed solvent of EtOAc and petroleum ether (5 : 3) to obtain a white or light yellow solid. 1 H NMR(400MHz,DMSO-d6),δ(ppm):7.13(s,1H,CH),7.03(s,1H,...
Embodiment 2
[0043] 1. Synthetic route:
[0044]
[0045] 2. The chemical structural formula of the product:
[0046]
[0047] 3. Preparation method:
[0048] 2 g of toluene 2,4-diisocyanate (11.48 mmol) was dissolved in 20 ml of dry acetone or acetonitrile or dry toluene and 10% (w / w) triethylamine or NaOH or DBU, and added 2,6- Di-tert-butyl-p-cresol (8.86 g, 40.19 mmol). The mixture was stirred at room temperature for 20 minutes, stirred at 50 degrees Celsius for 18 hours, cooled to room temperature, added petroleum ether (10ml) and stirred at room temperature for 10 minutes, filtered the precipitated solid, and reconstituted in a mixed solvent of ethyl acetate-petroleum ether (1:1). Crystallization gave 3.97 g of white powder, with a yield of 65.0%.
[0049] 1 H NMR (400MHz, DMSO-d6), d (ppm): 7.16-7.41 (m, 3H, 3CH), 6.91 (s, 2H, 2CH), 6.69 (s, 2H, 2CH), 3.35 (s, 3H, CH3), 2.50 (m, DMSO), 2.28 (s, 3H, CH3), 2.18 (s, 3H, CH3), 1.31-1.42 (m, 36H, 12CH3).
Embodiment 3
[0051] 1. Synthetic route:
[0052]
[0053] 2. The preparation method of 1,6-hexamethylene diisocyanate (intermediate 1):
[0054] Dissolve 5 g of 1,6-hexanediamine (43.03 mmol) in 20 ml of dry toluene or dichloromethane or THF or acetone, cool with ice water until 0-5 degrees Celsius, add trichlorodimethyl carbonate dropwise under nitrogen protection The solution (1:5, w / v), was stirred for 20 minutes, and 4.4 g (43.08 mmol) of triethylamine was added dropwise. The mixture was stirred at 0-10°C for 30 minutes, warmed to room temperature and stirred for 1 hour. Add 20ml EtOAc, wash the organic phase with 0.1N ice-water hydrochloric acid (20ml x3), and wash once with NaCl aqueous solution. The organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated (yield 96%) and directly used in the next reaction.
[0055] 3. The chemical structural formula of the product:
[0056]
[0057] 4. The preparation method of the product:
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com