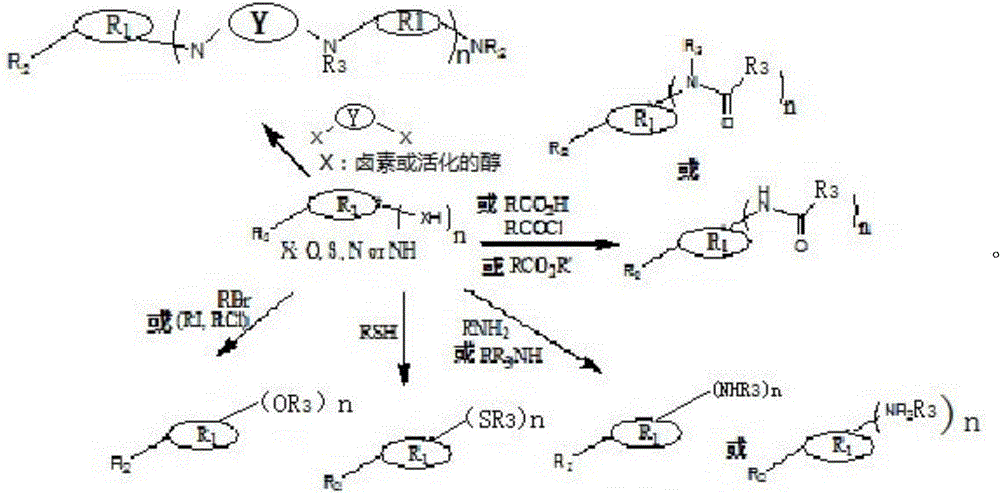
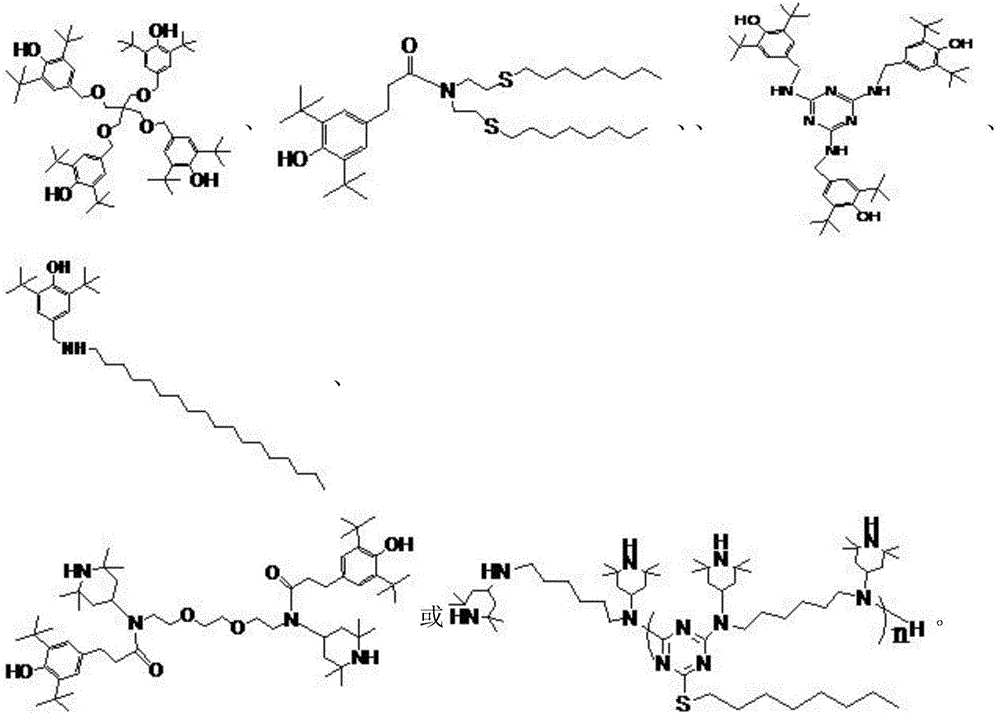
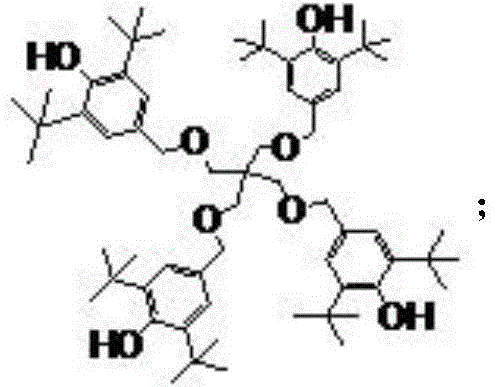
A kind of preparation method of multifunctional synergistic antioxidant stabilizer
An antioxidant stabilizer, multi-functional technology, applied in the preparation of organic compounds, chemical instruments and methods, preparation of aminohydroxy compounds, etc., can solve problems such as damage, color damage, embrittlement and cracking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Structural formula
[0038]
[0039] 2. Synthetic route:
[0040]
[0041] 3. Synthesis step 1 of 4-bromomethyl-2,6-di-tert-butylphenol (intermediate 1):
[0042] 5 g of 2,6-di-tert-butyl-p-cresol (22.69 mmol) dissolved in CCl 4 or CHCl 3 Or dichloromethane or THF or toluene and other solvents (20-50 milliliters), under nitrogen protection and ultraviolet lamp (mercury lamp 350 watts) irradiation, drop liquid bromine (1.2-1.5 mmol) above solvent solution (15-50 milliliter), the rate of addition can be determined with the speed of the reaction, and TLC monitors the reaction process.
[0043] Stir for 5-20 minutes after the titration, remove the organic solvent under vacuum, and use the oily product directly in the next step.
[0044] 4. Synthesis step 2 of 4-bromomethyl-2,6-di-tert-butylphenol (intermediate 1):
[0045] 5.0 g of 2,6-di-tert-butyl-p-cresol (22.69 mmol) was dissolved in CCl 4 or CHCl 3 Or in solvents (25-50 milliliters) such as dichlorometh...
Embodiment 2
[0050] 1. Structural formula
[0051]
[0052] 2. Synthetic route:
[0053]
[0054] 3. Preparation of Intermediate 1:
[0055] Add 6.1 g of mercaptan (42.30 mmol) dropwise to 3 g of 2,2-dichloroethylamine (21.12 mmol) and 10-50% KI of THF or acetone or acetonitrile or dichloromethane or ethanol, etc. (1 : 5-20, w / v) solution, the mixture was heated to 40-70° C., reacted for 3-9 hours, TLC monitored the reaction progress until the reaction was complete. The organic solvent was removed in vacuo, NaCl aqueous solution and dichloromethane or ethyl acetate were added, mixed well, the organic phase was separated, and the aqueous phase was extracted twice. Anhydrous Na 2 SO 4 The organic phase was dried, filtered and intermediate 1 was used directly in the next step after concentration.
[0056] 4. Preparation of Intermediate 2:
[0057] 5.93 g of 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid (21.30 mmol) was dissolved in dry THF or dichloromethane or ethyl acetate...
Embodiment 3
[0062] 1. Structural formula
[0063]
[0064] 2. Synthetic route:
[0065]
[0066] 3. Synthesis step 1 of 2,2,6,6-di-tert-butyl-4-bromomethylphenol (intermediate 1):
[0067] 5 grams of 2,2,6,6-di-tert-butyl-p-cresol (27.11 mmol) was dissolved in CCl4 or CHCl3 or CH2Cl2 or THF or chlorobenzene or toluene or dibromoethane solvent (1:5-20, w In / v), under the protection of nitrogen, a solution of the same volume of bromine (28.01-37.57 mmol) was added dropwise under the irradiation of a 350-watt mercury lamp, and the reaction progress was monitored by TLC. After the dropwise addition, the mixture was stirred for 5-40 minutes, and the organic solvent was removed under vacuum to obtain a light reddish-brown oil, which was directly used in the next reaction, with a yield of 95-100%.
[0068] 4. Synthesis step 2 of 2,2,6,6-di-tert-butyl-4-bromomethylphenol (intermediate 1):
[0069] 5 g of 2,2,6,6-di-tert-butyl-p-cresol (27.11 mmol) dissolved in CCl 4 or CHCl 3 or CH 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com