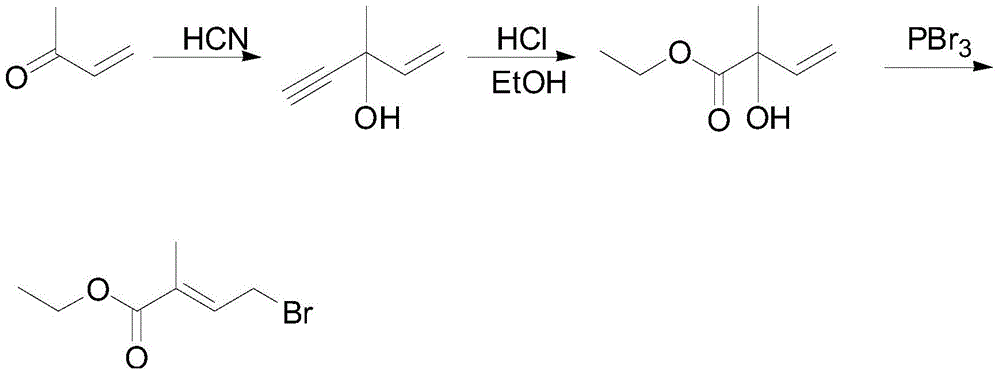
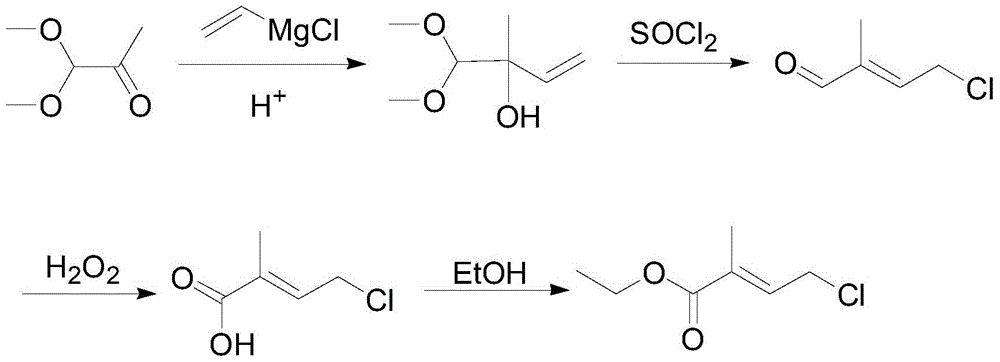
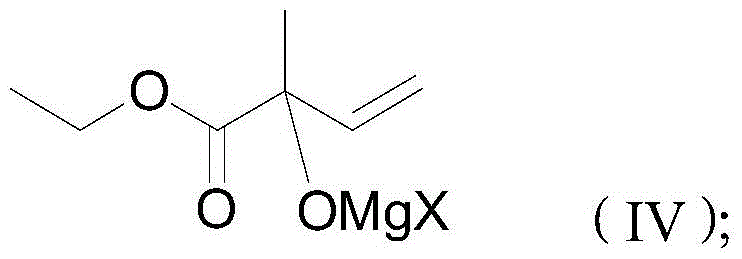The preparation method of 4-halo-2-methyl-2-butenoic acid ethyl ester
A technology of ethyl crotonate and ethyl pyruvate, which is applied in the field of chemical intermediate synthesis, can solve the problems of low total reaction yield and long process route, and achieve good reaction selectivity, simple overall process and low equipment requirements harsh effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A. Preparation of hydroxy compound (V)
[0033] Add 23.43g ethyl pyruvate (purity 99%) and 80ml toluene into a 250ml four-necked flask, stir and cool down to -10°C, start to add 91ml of vinylmagnesium chloride Grignard reagent (content 2.2mol / L, commercially available) The product, the solvent is tetrahydrofuran), the drip is completed in about 60 minutes, and the reaction is kept for 1 hour. After heat preservation, the product obtained is the condensate.
[0034] The condensate was slowly added dropwise to 150ml of dilute sulfuric acid (10% by mass), the reaction temperature was kept within 50°C during the dropping process, and the temperature keeping reaction was continued for 10 minutes after dropping. After standing for layering, the upper organic phase was separated, and the lower aqueous phase was extracted twice with toluene, with 30 ml of toluene each time. Combine and extract toluene to the organic phase, neutralize it with 100ml saturated sodium bicarbonate, sep...
Embodiment 2
[0038] A. Preparation of hydroxy compound (V)
[0039] Add 23.43g ethyl pyruvate (purity 99%) and 80ml toluene into a 250ml four-necked flask, stir and cool to -10℃, start to add 87ml vinylmagnesium bromide Grignard reagent (content 2.3mol / L, Commercially available product, the solvent is tetrahydrofuran), the drip is completed in about 60 minutes, and the reaction is kept for 1 hour. After heat preservation, the product obtained is the condensate. The condensate was slowly added dropwise to 150ml of dilute sulfuric acid (with a concentration of 10% by mass), the reaction temperature was kept within 50°C during the dropping process, and the temperature keeping reaction was continued for 10 minutes after dropping. After standing for layering, the upper organic phase was separated, and the lower aqueous phase was extracted twice with toluene, with 30 ml of toluene each time. Combine and extract toluene to the organic phase, neutralize it with 100ml saturated sodium bicarbonate, s...
Embodiment 3
[0043] A. The hydroxyl compound (V) was prepared in the same way as in Example 1
[0044] B. Preparation of bromocarbon five ester (I)
[0045] Add 22.85g of hydroxy compound (V) (purity 94.5%) and 60ml of dichloromethane into a 250ml four-necked flask, stir and cool the reaction mixture to 0°C, and start adding 32.0g of hydrobromic acid (mass percentage concentration 57%). , The drip is finished in about 30min, and the reaction is kept for 20min. Let stand to separate the layers, separate the organic phase, and extract the aqueous phase with 20 ml of dichloromethane and combine into the organic phase. The organic phase was neutralized with 80ml saturated sodium bicarbonate and then washed once with 80ml saturated sodium chloride. After washing, the dichloromethane was recovered to obtain 28.7g concentrate, which was compared and analyzed by GC with the commercially available standard bromocarbon pentacarboxylate (I) , The purity is 91.8%, and the yield is 84.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com