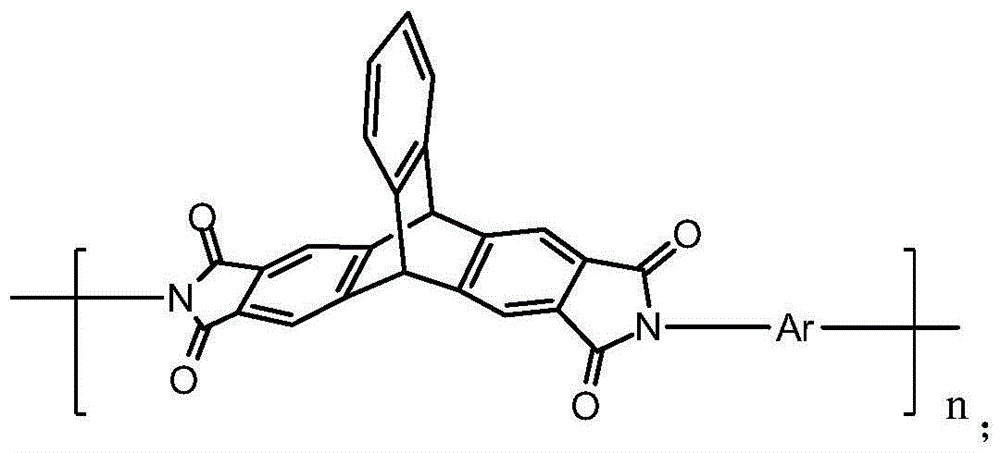
Polyimide based on 2,3,6,7-triptycene tetracarboxylic dianhydride and preparation method of polyimide
A technology of tetra-acid dianhydride polyimide and pterene tetra-acid dianhydride is applied in the field of polyimide and its preparation based on 2,3,6,7-triptylidene tetra-acid dianhydride, achieving easy Control, increase glass transition temperature, reduce the effect of force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The present invention is based on a preparation method of 2,3,6,7-triptycene-tetraacid dianhydride polyimide, the preparation method comprising the following steps:
[0030] (1) With o-xylene and dichloromethane under the catalysis of anhydrous aluminum trichloride, 2,3,6,7-tetramethylanthracene is generated after heating;
[0031] (2) Diazotization of 2,3,6,7-tetramethylanthracene and anthranilic acid in the presence of isoamyl nitrite to diazotize the benzyne produced in situ for Diels-Alder reaction, then the crude product 2,3, 6,7-Tetramethyltriptycene;
[0032] (3) The crude product 2,3,6,7-tetramethyltriptycene is separated by chromatography and washed with a mixed eluent to obtain 2,3,6,7-tetramethyltriptycene with a purity greater than 99%;
[0033] (4) 2,3,6,7-tetramethyltriptycene is oxidized under reflux conditions in a mixed solvent of potassium permanganate and pyridine-water to generate 2,3,6,7-triptycene tetracarboxylic acid;
[0034] (5) 2,3,6,7-tripty...
Embodiment 1
[0044] Under the conditions of ice-salt bath and 400r / min mechanical stirring, in a 250mL three-neck flask with 120mL o-xylene and 70mL methylene chloride, add 60g aluminum trichloride in four times, and keep the system temperature at 0°C during the addition process; After the addition, react at room temperature for 0.5h, then react in a water bath at 65°C for 4h; then slowly pour the reaction product into 300mL of 5% hydrochloric acid ice-water mixture, stir during the pouring process, then stand still and filter with suction (The filter cake is washed with anhydrous acetone) and dried to obtain 20 g of white sheet-like 2,3,6,7-tetramethylanthracene solid, whose melting point is 299 ° C, and its structure is obtained through 1 Confirmation by HNMR characterization.
[0045] 1 HNMR (400MHz, CDCl3): δ = 2.43 (s, 12H), 7.68 (s, 4H), 8.13 (s, 2H).
[0046] Weigh 2g of 2,3,6,7-tetramethylanthracene and add it into a 500mL three-neck flask with 100mL of dichloroethane, heat to re...
Embodiment 2
[0068] Under the conditions of ice-salt bath and 500r / min mechanical stirring, in a 250mL three-necked flask with 120mL o-xylene and 70mL methylene chloride, add 73g aluminum trichloride in four times, and keep the system temperature at -5°C during the addition process ; After the addition, react at room temperature for 0.75h, and then react in a 60°C water bath for 3h; then slowly pour and disperse the reaction product into 300mL of 5% hydrochloric acid ice-water mixture, stir during the pouring process, and then let it stand , suction filtration (the filter cake is washed with anhydrous acetone), and dried to obtain 24 g of white flakes, that is, 2,3,6,7-tetramethylanthracene solid, whose melting point is 299 ° C, and its structure is obtained through 1 Confirmation by HNMR characterization.
[0069] 1 HNMR (400MHz, CDCl3): δ = 2.43 (s, 12H), 7.68 (s, 4H), 8.13 (s, 2H).
[0070] Weigh 2g of 2,3,6,7-tetramethylanthracene and add it into a 500mL three-neck flask with 100mL o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com