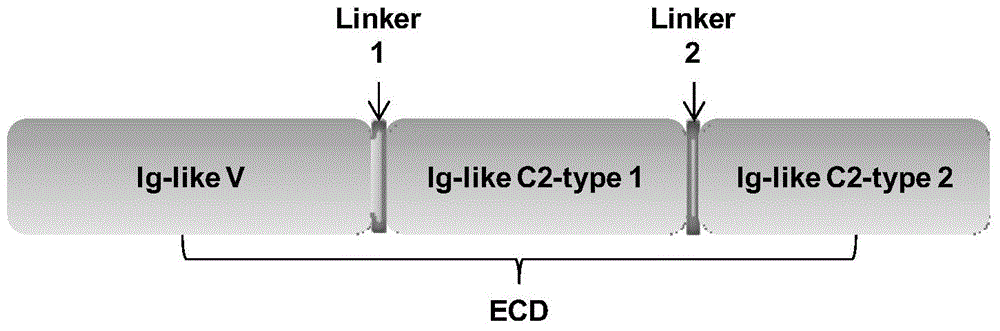
Application of human Nectin-2 protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]Example 1 Obtaining of human Nectin-2 protein
[0034] According to the human Nectin-2 mRNA sequence published in GenBank (Accession No.: CR456818.1), the 1-1035th nucleotide sequence was spliced with the gene sequence of the immunoglobulin Fc fragment. The spliced gene sequence is shown in SEQ ID No.5 is shown.
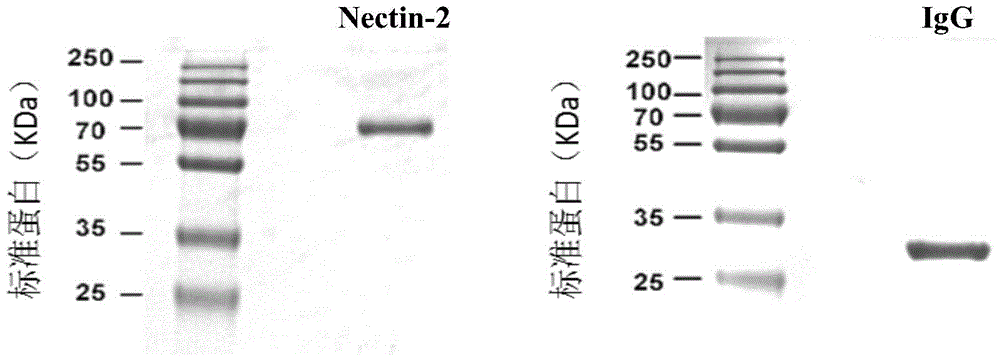
[0035] After the above sequence was artificially synthesized, it was cloned into the mammalian expression vector pcDNA3.1, and the constructed expression vector was transiently transferred into HEK293 cells to realize the expression of the fusion protein and purify the target protein. The results of SDS-PAGE gel electrophoresis after protein purification are as follows: figure 2 shown.
[0036] The specific process of cell transfection, cell culture and protein purification is as follows: First, HEK293 cells were cultured in suspension in 293FreeStyle medium (GIBCO) in 5% CO 2 Cultivate under conditions, the speed of the carbon dioxide shaker (INFORS) i...
Embodiment 2
[0037] Example 2 Application of Human Nectin-2 Protein in Suppressing Obesity
[0038] In order to verify the anti-obesity effect of the Nectin-2 protein prepared in Example 1, the following high-fat diet-induced obesity mouse model was constructed in this example:
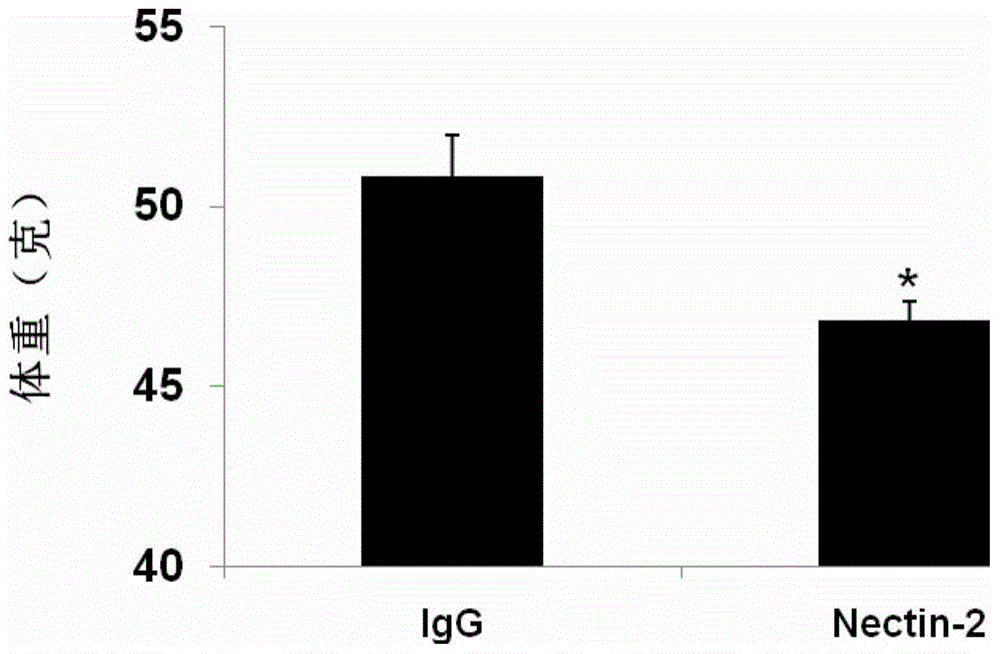
[0039] 6-week-old C57BL / 6J male mice were fed with 60Kcal% high-fat diet (Research diet, D12451), and body weight was recorded every week. After 8 weeks of induction and waiting for their obesity, the high-fat diet-induced obesity group was randomly divided into a control group (IgG, 10) and an experimental group (Nectin-2, 10); then the experimental group was treated with Nectin-2 (Example 1) treated with subcutaneous injection at a dose of 5 mg / kg body weight / week; and the control group was treated with the same dose of IgG (immunoglobulin Fc as the control protein), administered by subcutaneous injection, administered weekly 2 weeks before the administration, the average daily food intake of the animals in eac...
Embodiment 3
[0041] Example 3 Application of Human Nectin-2 Protein in Lowering Fasting Blood Glucose
[0042] Existing research results have shown that obesity is often accompanied by severe insulin resistance. In the present embodiment, the following experiments were further carried out on the mice administered in the 9th week in Example 2:
[0043] After 9 weeks of administration in Example 2, the mice were fasted overnight to measure the fasting blood glucose level.
[0044] The result is as Figure 4 As shown, the fasting blood glucose (5.84mmol) of the control group (IgG) induced by high-fat diet was significantly higher than that of the experimental group treated with Nectin-2 (5.2mmol, p<0.05).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com