Olefin polymerization catalyst composition and application method thereof
A technology of olefin polymerization and composition, which is applied in the field of olefin polymerization catalyst composition, can solve problems such as complex preparation methods, and achieve the effects of simple catalytic system, low environmental hazards, easy preparation and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
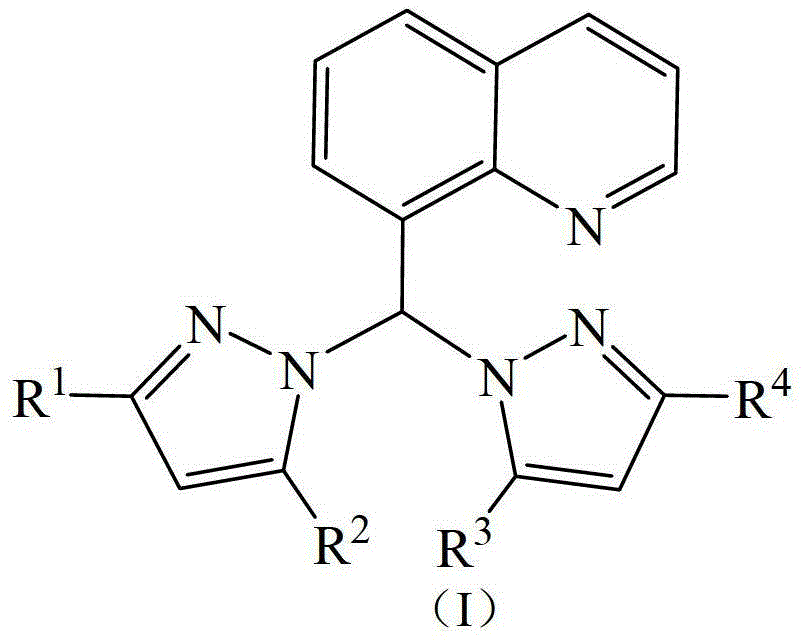
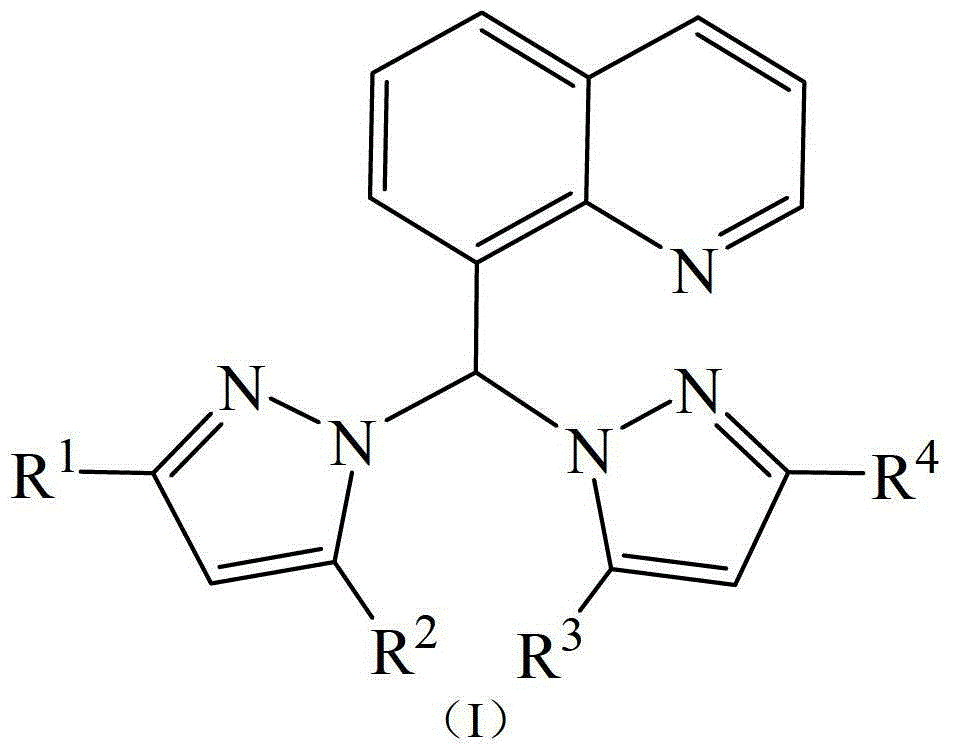
[0028] The synthesis of ligand A, ligand A is the ligand shown in formula I, wherein R 1 =R 2 =R 3 =R 4 =CH 3 .
[0029] The ligand preparation method is: in a dry reactor, add NaH (1.2g, 30mmol), 30ml of anhydrous tetrahydrofuran in a Schlenk tube, slowly add 3,5-dimethylpyrazole (2.8g, 30mmol ), release gas, stir for 30 minutes, add thionyl chloride (1ml, 15mmol), the brown-gray transparent reaction solution turns off-white, remove the ice-water bath, stir for 30 minutes at room temperature, add quinoline aldehyde (2.4g, 15mmol), Pyridine (1.2g, 15mmol), refluxed in an oil bath at 80°C for 6 hours, quenched with saturated aqueous sodium bicarbonate solution, extracted three times with ethyl acetate, and purified by column chromatography to obtain a white solid, Ligand A (3.5g, yield 71wt% ).
[0030] 1 H-NMR (δ, ppm, CDCl 3 , TMS): 8.8~7.4 (m, 6H, Quinoline-H), 8.7 (s, 1H, CH), 5.8 (s, 2H, Pyrazole-H), 2.23 (s, 6H, CH 3 ), 2.18(s, 6H, CH 3 ).
Embodiment 2
[0032] The synthesis of ligand B, ligand B is the ligand shown in formula I, wherein R 1 =R 2 =R 3 =R 4 =H.
[0033] The preparation method is the same as Ligand Synthesis Example 1, except that 3,5-dimethylpyrazole is replaced by pyrazole, and other conditions remain unchanged. Yield 74wt%. 1 H-NMR (δ, ppm, CDCl 3 , TMS): 8.8-7.3 (m, 12H, Quinoline-H and Pyrazole-H), 8.7 (s, 1H, CH).
[0034] Polymerization example
Embodiment 3
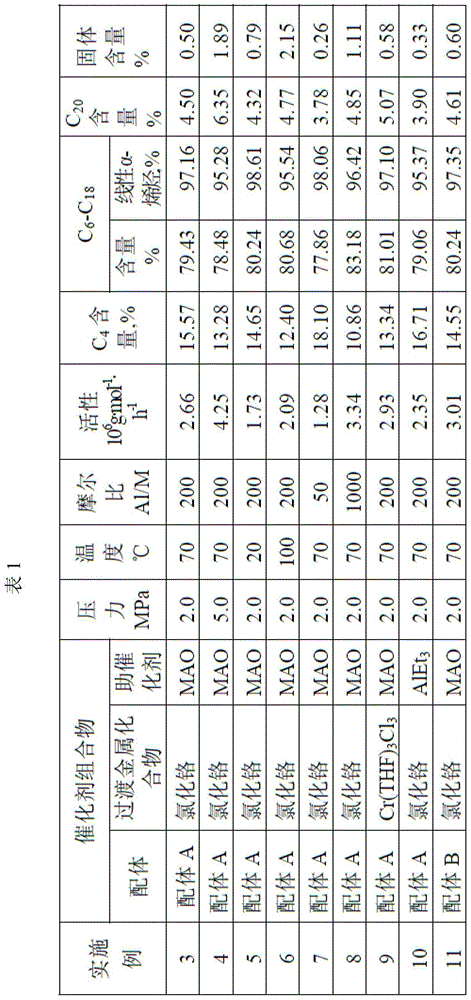
[0036]A 300mL stainless steel polymerization kettle is used. The autoclave was heated to 100°C, evacuated and replaced with nitrogen for several times, and then filled with ethylene until the pressure of ethylene was 2 MPa and cooled to room temperature. Then add dehydrated toluene at 70°C, and at the same time add 10 μmol of the ligand (ligand A), chromium chloride and methylaluminoxane (MAO) in Example 1. The total volume of the mixed solution is 100 mL, in which the ligand, The molar ratio of chromium chloride and methylaluminoxane is 1:1:200, that is, the amount of chromium chloride added is 10 μmol, the amount of methylaluminoxane added is 2 mmol, the reaction pressure is controlled at 2.0 MPa, and ethylene is introduced to carry out ethylene oligomerization reaction.
[0037] After the reaction was completed, the system was cooled to room temperature, the gas phase product was collected in a gas metering tank, and the liquid phase product was collected in an Erlenmeyer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com