Preparation method of thermo-sensitive chitosan polymer connected with sugar molecules
A chitosan and temperature-sensitive technology, applied in the field of biological materials and nanomaterials, can solve problems such as single function, and achieve the effects of wide source of raw materials, simple and easy synthesis method, and wide application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
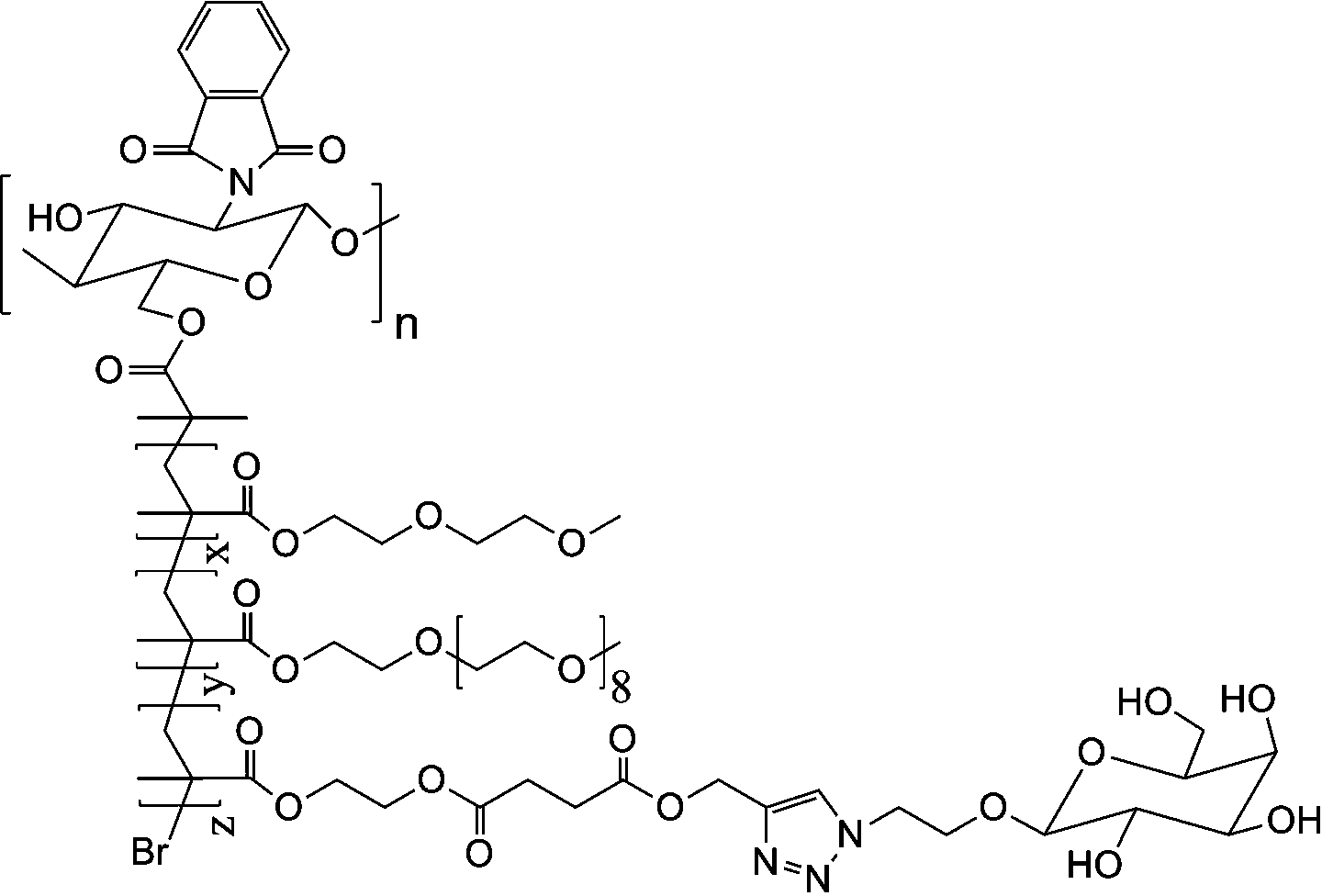
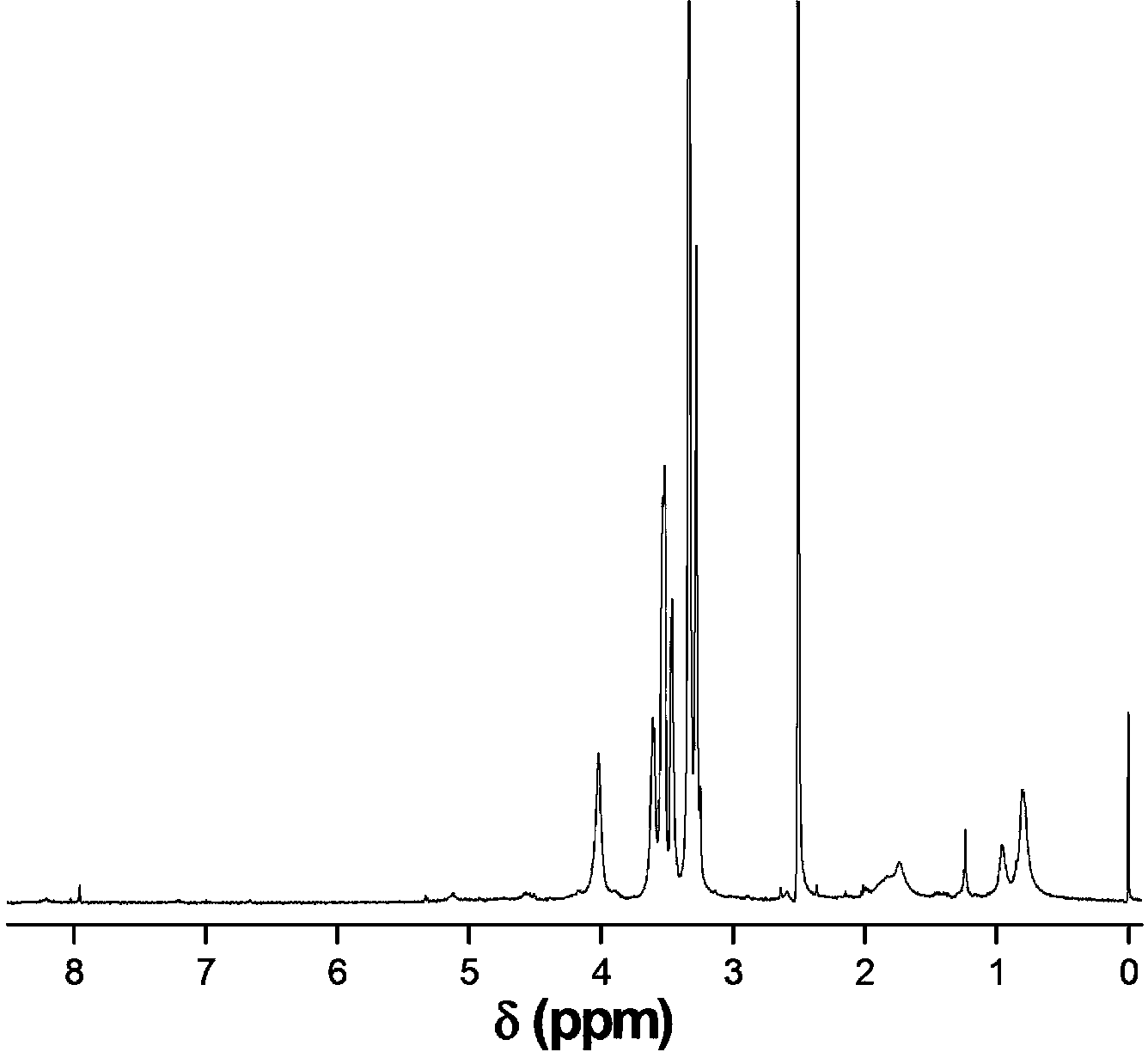
[0029] Weigh 0.6 g of CS-Br, dissolve in N,N-dimethylformamide, add MEO 2 MA monomer 5.2 grams, OEGMA monomer 0.3 grams, add catalyst cuprous chloride (80 mg) / pentamethyl divinyl triamine (40 mg) again, system is through vacuumizing-argon process three times, in argon 40 under air protection o C for 18 hours, dialyzed and freeze-dried to obtain CS- g -P(MEO 2 MA- co -OEGMA)-Br. Weigh out 3.6 g CS- g -P(MEO 2 MA- co -OEGMA)-Br, dissolved in N,N-dimethylacetamide, add 2.0 g of HEMA monomer, then add catalyst cuprous chloride (48 mg) / bipyridine (105 mg), the system is vacuum- Nitrogen filling process three times, under the protection of nitrogen at 60 o C for 10 hours, dialyzed and freeze-dried to obtain CS- g -P(MEO 2 MA- co -OEGMA)- b -PHEMA. Weigh out 2.8 g CS- g -P(MEO 2 MA- co -OEGMA)- b -PHEMA, dissolved in N,N-dimethylformamide, added 2.4 grams of propargyl-3-carboxypropionate, 3.2 grams of N,N-dicyclohexylcarbodiimide, 20 o C for 48 hours, suction fi...
Embodiment 2
[0031] Weigh 0.6 g of CS-Br, dissolve in N,N-diethylformamide, add MEO 2 MA monomer 4.8 grams, OEGMA monomer 0.7 grams, add catalyst cuprous bromide (111 mg) / pentamethyl divinyl triamine (40 mg) again, system is through vacuumizing-argon process three times, in argon Under the protection of gas at 45 o C for 16 hours, dialyzed and freeze-dried to obtain CS- g -P(MEO 2 MA- co -OEGMA)-Br. Weigh out 2.4 g CS- g -P(MEO 2 MA- co -OEGMA)-Br, dissolved in N,N-dimethylformamide, add HEMA monomer 1.5 g, then add catalyst cuprous chloride (52 mg) / pentamethyldivinyltriamine (26 mg) , the system was vacuum-filled with argon three times, under the protection of argon at 45 o C for 18 hours, dialyzed and freeze-dried to obtain CS- g -P(MEO 2 MA- co -OEGMA)- b -PHEMA. Weigh out 2.0 g CS- g -P(MEO 2 MA- co -OEGMA)- b -PHEMA, dissolved in N,N-dimethylformamide, added 1.2 grams of propargyl-3-carboxypropionate, 0.9 grams of chlorosulfonic acid, 25 o C for 40 hours, suction...
Embodiment 3
[0033] Weigh 0.6 g of CS-Br, dissolve in N,N-dimethylformamide, add MEO 2 6.0 grams of MA monomer, 1.3 grams of OEGMA monomer, and then add catalyst cuprous bromide (110 mg) / hexamethyltriethylenetetramine (103 mg), and the system is vacuumized-argon-filled three times. Under the protection of air at 50 o C for 12 hours, dialyzed and freeze-dried to obtain CS- g -P(MEO 2 MA- co -OEGMA)-Br. Weigh out 3.9 g CS- g -P(MEO 2 MA- co -OEGMA)-Br, dissolved in N,N-dimethylacetamide, adding 1.8 grams of HEMA monomer, and then adding the catalyst cuprous bromide (48 mg) / bipyridine (108 mg), the system was vacuum- Argon filling process three times, under the protection of argon at 55 o C for 12 hours, dialyzed and freeze-dried to obtain CS- g -P(MEO 2 MA- co -OEGMA)- b -PHEMA. Weigh out 3.2 g CS- g -P(MEO 2 MA- co -OEGMA)- b -PHEMA, dissolved in N,N-diethylformamide, added 1.3 grams of propargyl-3-carboxypropionate, 1.0 grams of thionyl chloride, 30 o C for 32 hours, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com