Method for production of streptavidin labeled phycocyanin fluorescent protein and application
A streptavidin and phycocyanin technology, which is applied in the field of producing streptavidin-labeled phycocyanin fluorescent proteins, can solve the problems of reduced fluorescence and biological activity of fusion proteins, difficulty in experimental synchronization control of expression levels, and plasmids. Loss and other problems, to achieve good practical value and application prospects, reduce production costs, and improve the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the construction of genetic engineering strain
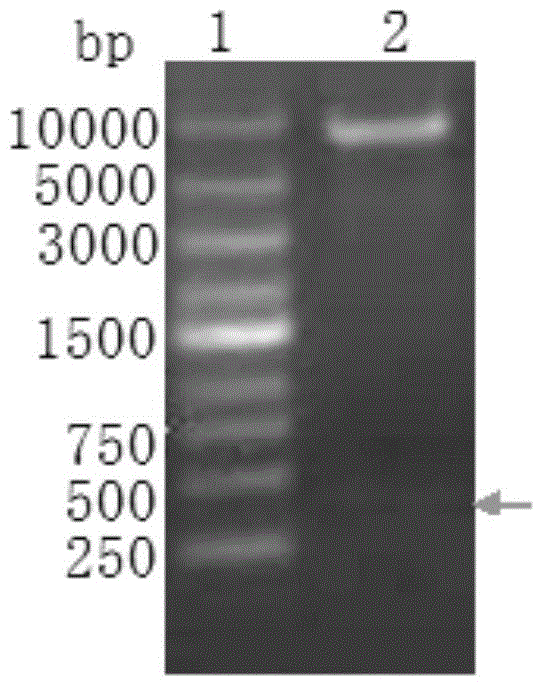
[0032] According to the information in the international gene database, referring to the Chinese invention patent: a preparation method of high-stability phycocyanin fusion fluorescent protein (publication number: CN101838661A), various genes for expression plasmid construction were cloned. The phycocyanin-like apoprotein subunit gene (cpcA), the core streptavidin gene (sa), and the genes encoding phycocyanin cleavage isomerase E and F (cpcE and cpcF) were cloned into the same promoter Next; the heme oxygenase 1 gene (hox1) or its homologous gene, the phycocyanin ferredoxin reductase gene (pcyA) or its homologous gene are placed under another promoter to construct the expression plasmid pAC, After conventional enzyme digestion detection and sequencing analysis, the plasmid was constructed correctly ( figure 1). The pAC was transformed into the host strain BL21(DE3) to obtain the genetically engineered stra...
Embodiment 2
[0034] Example 2: BAC Shake Flask Culture and Induced Expression of Recombinant Protein
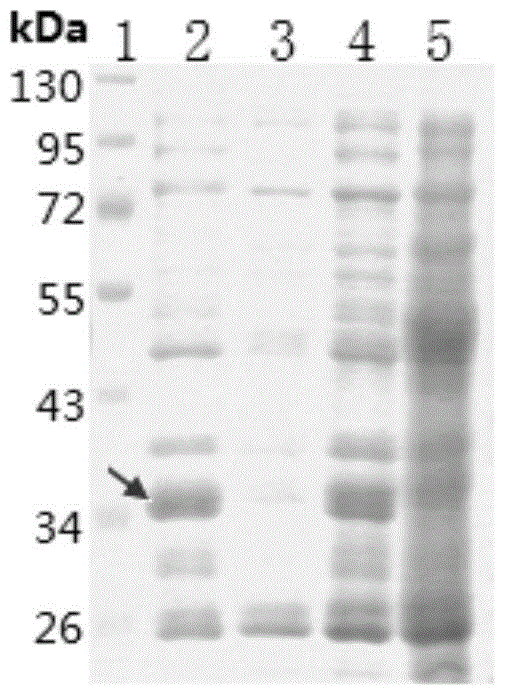
[0035] According to the conventional shaking flask culture conditions, different carbon sources were added, and the BAC was cultured to the pre-logarithmic growth stage. Add inducers with final concentrations of 0.2 and 1 mmol / L respectively, induce expression at 18-37°C, 100-350 rpm for 8 hours, collect cells, and observe the color change of cell pellets. As can be seen from Table 1, adding 0.4% glycerol (or 1% glucose) accounting for the weight of the medium in the medium, and when selecting 28°C, when adding an inducer with a final concentration of 5.5mmol / L lactose, the expression level of the target protein is the highest. it is good. However, SDS-PAGE analysis found that most of the recombinant protein existed in the precipitate in the form of inclusion bodies ( figure 2 ).
[0036] Table 1 Effects of different types and concentrations of inducers on the expression of target pro...
Embodiment 3
[0038] Embodiment 3: Purification and preparation of recombinant protein
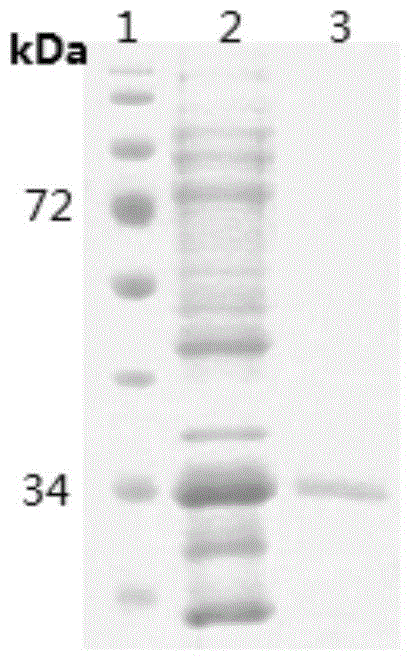
[0039] The recombinant protein contains a histidine tag, and the protein is purified using a nickel affinity column. The specific operation steps are: after BAC is induced to express, use denaturing buffer (20-50mM Tris-HCl buffer, 8M (mol / L) urea Urea, pH7.3-8.3, 0.15M (mol / L) NaCl, the balance is PBS phosphate-buffered saline), crushed at room temperature for 15-30 minutes, centrifuged, and the supernatant was filtered through a 0.45 μm filter membrane, and then affinity purified with a nickel column. After SDS-PAGE analysis, the purified recombinant protein was dialyzed against 10 L of 0.1 M phosphate buffer (TAKARA) at 4° C. for 48 hours. The purified recombinant protein had only one band, and the size was consistent with the expectation ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com