An injectable hydrogel with controlled release capability for tissue engineering and its application
A tissue engineering and injection technology, applied in the field of biomedical engineering materials, can solve problems such as poor mechanical properties of collagen gels, achieve good biocompatibility and degradability, excellent controllability, gel time controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The synthesis of embodiment 1 gelatin-tyramine-heparin complex
[0051] The concrete steps of synthetic method are as follows:
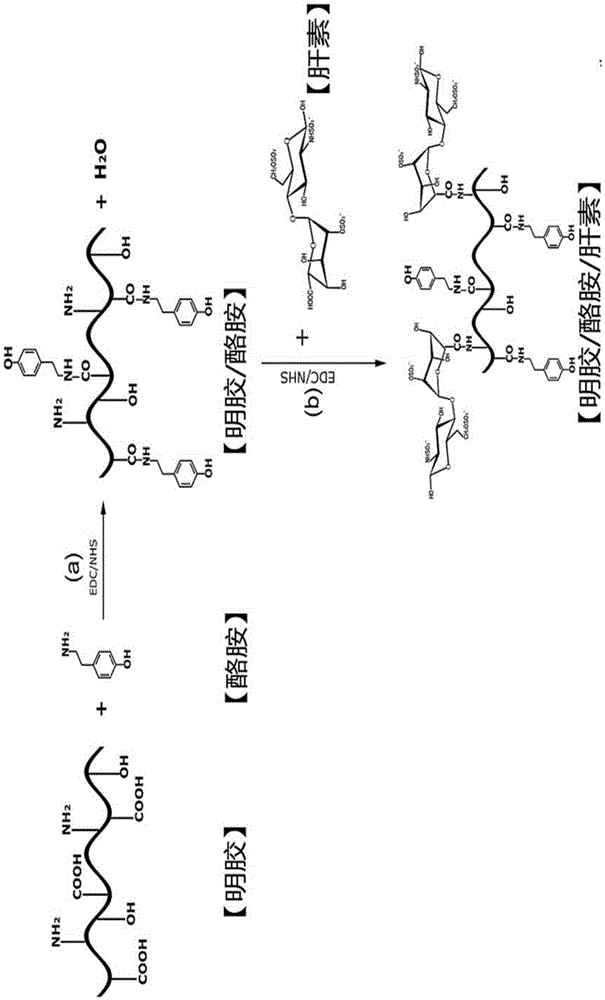
[0052] (1) Synthesis of gelatin-tyramide complex
[0053] Weigh 4g of B-type gelatin and dissolve it in 50mM 2-(N-morpholine)ethanesulfonic acid (MES) solution, heat to 60°C to fully dissolve it, then cool to room temperature, weigh 1g of tyramine and dissolve it in the above solution, and Add cross-linking agent (EDC / NHS) and stir at room temperature for 12-24 hours; put the reacted solution into a dialysis bag with a molecular weight cut-off of 10,000 and dialyze in deionized water for 2-3 days, freeze-dry the sample after the dialysis, Obtain gelatin-tyramide complex;
[0054] (2) Synthesis of gelatin-tyramine-heparin complex
[0055] Weigh 4 g of the gelatin-tyramine complex prepared in step (1) and dissolve it in 50 mM 2-(N-morpholine) ethanesulfonic acid (MES) solution, heat to 60° C. to fully dissolve it, then cool to room temperatur...
Embodiment 2
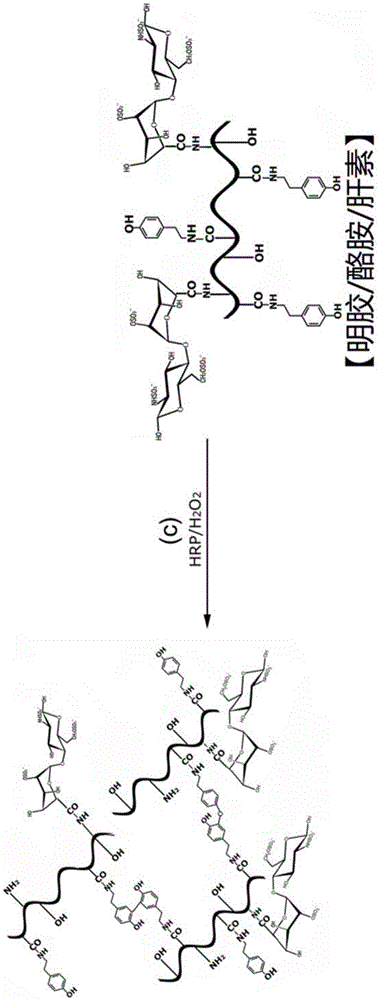
[0057] Example 2 Preparation of Injectable Hydrogel with Controlled Release Capability for Tissue Engineering
[0058] Dissolve the gelatin-tyramine-heparin complex synthesized in Example 1 in deionized water to form a gel solution with a mass fraction of 2%, add 1 unit / ml of HRP, mix well, and then add 1mM H 2 o 2 , forming a hydrogel within five minutes, the chemical reaction formula of the preparation method is as follows figure 2 As shown, the hydrogel can be injected using a syringe.
Embodiment 3
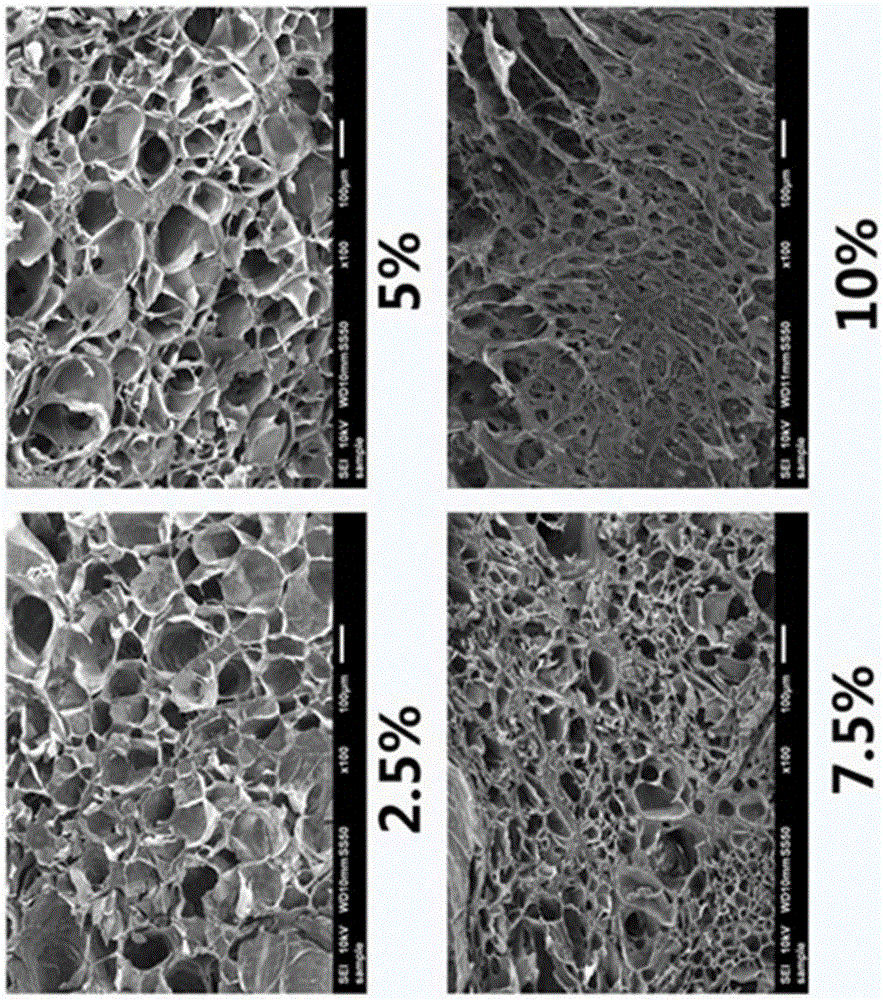
[0059] Example 3 Effect of polymer (gelatin-tyramine-heparin complex) concentration on hydrogel pores
[0060] Dissolve the gelatin-tyramine-heparin complexes synthesized in Example 1 in deionized water to form gel solutions with mass fractions of 2.5%, 5%, 7.5%, and 10%, respectively, and then add 1 unit / ml HRP, mix well, then add 1mM H 2 o 2 , the hydrogel can be formed, and the hydrogel pores formed by gel solutions with different mass fractions are as follows image 3 shown. The mechanical strength of hydrogels can be tuned by changing the degree of crosslinking, such as Figure 4 The appearance of hydrogels with different mechanical strengths is given in .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com