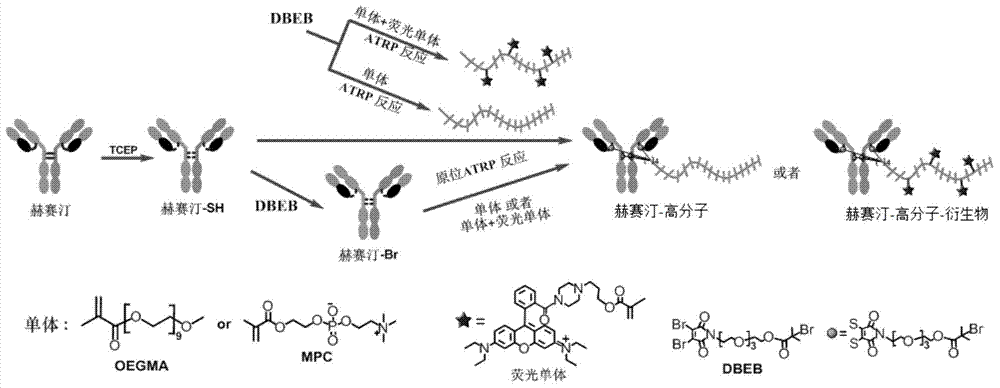
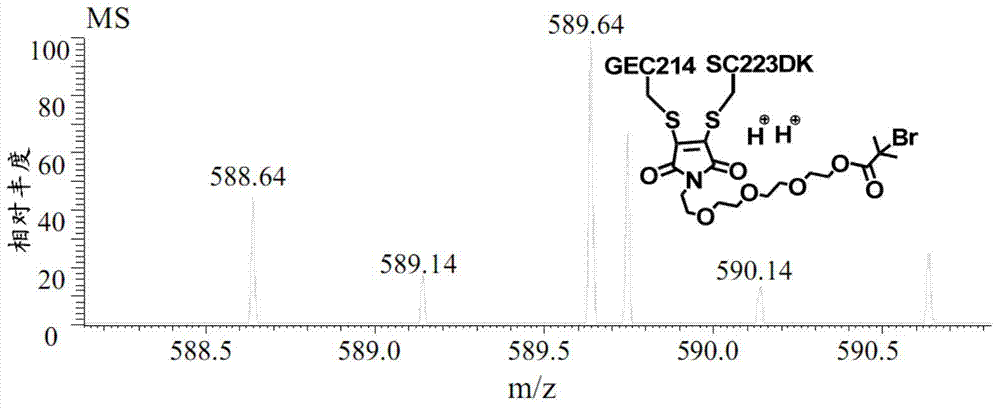
Antibody-polymer combined body and fluorescent derivative thereof and preparation method of antibody-polymer combined body and fluorescent derivative
A technology of polymer conjugates and polymer monomers, applied in the field of biomedicine, can solve problems such as difficult separation of products, difficulty in maintaining activity, poor quality control, etc., and achieve the effect of simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070]Specifically, the preparation method may include two methods: the first is an in-situ polymerization method, and the method includes: a1) preparing an antibody-initiator conjugate, wherein the elicitor is bound to the disulfide of the antibody bond or free sulfhydryl group; b1) the antibody-initiator conjugate is mixed with a polymer monomer or a polymer monomer and a fluorescent monomer in a buffer, and triggers the polymerization of the polymer monomer under the action of a catalyst And the antibody-macromolecule conjugate or the fluorescent derivative of the antibody-polymer conjugate is prepared. The second is the grafting method. The grafting method pre-prepared the polymer, and then coupled with the specific site of the antibody to prepare the antibody-polymer conjugate and its fluorescent derivatives. The method includes: a2) the initiator is placed in the catalyst Initiate the polymerization of polymer monomers or polymer monomers and fluorescent monomers under t...
Embodiment 1
[0095] 1. Preparation of ATRP initiator DBEB (chemical formula I-2).
[0096]
[0097] Preparation of 3,4-dibromo-1-((2-(2-(2-hydroxyethoxy)ethoxy)ethoxy)methyl)-1H-pyrrole-2,5-dione (chemical formula I-1): Dibromomaleimide (1.02g, 4mmol) was dissolved in 20ml of dry tetrahydrofuran, and triphenylphosphine (1.05g, 4mmol) and diisopropyl azodicarboxylate were slowly added at room temperature (DIAD, 790 μL, 4 mmol) and tetraethylene glycol (1.4 ml, 8 mmol) and stirred for 20 hours. Use a rotary evaporator to remove the solvent, dry loading, and flash column chromatography to obtain the compound (yellow oil, 398mg, yield rate 23%) described in the chemical formula I-1 provided by the invention, and reclaimed 50% of the dibromo Substitute maleimide.
[0098] The compound of chemical formula I-1 is a yellow oil. 1 H NMR (CDCl 3 , 400MHz) δ3.59-3.71(m, 14H), 3.81-3.84(m, 2H). 13 C NMR (CDCl 3 , 100MHz) δ39.0, 61.8, 67.7, 70.1, 70.4, 70.6, 70.8, 72.6, 129.5, 164.0. MS (IES) ...
Embodiment 2
[0126] 1. Preparation of rhodamine B derivative fluorescent monomer (formula I-6)
[0127]
[0128] 1) Synthesis of 3-bromopropyl methacrylate (chemical formula I-3).
[0129] Methacryloyl chloride (1.04g, 10mmol) and 3ml of triethylamine were slowly added dropwise to a mixed solution of 3-bromopropanol (1.39g, 10mmol) in dichloromethane (20ml) and DFM (1ml). After reacting at room temperature for 12 hours, 0.1M dilute hydrochloric acid was slowly added dropwise to the reaction solution until pH<7.0, extracted with ethyl acetate (3×25 mL), the organic phase was dried over anhydrous magnesium sulfate, filtered and concentrated. Flash column chromatography obtained the compound of chemical formula I-3 provided by the present invention (colorless oil, 1.13 g, 55%).
[0130] The compound of formula I-3 is a yellow oil. 1 H NMR (400MHz, CDCl 3 )δ1.95(s, 3H), 2.21-2.27(m, 2H), 3.49(t, 2H, J=6.4), 4.29(t, 2H, J=6.4), 5.58(s, 1H), 6.11( s, 1H). MS (ESI) m / z: 229 [M+Na] + .
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com