Antimicrobial peptide MP1106, preparation method and application thereof
A technology of MP1106 and antimicrobial peptide, which is applied in the field of genetic engineering and molecular biology, can solve the problems of high cytotoxicity, high production cost, and weak bactericidal activity, and achieve the effect of wide application prospects and high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The acquisition of embodiment 1 antimicrobial peptide MP1106 gene fragment
[0048] 1. Optimization of the gene sequence of the antimicrobial peptide MP1106
[0049] According to the yeast codon table: Pichia pastoris[gbpln]: 137 CDS's (81301 codons), from: www.kazusa.or.jp / codon / .
[0050]
[0051]
[0052] The amino acid sequence of MP1106 according to the design: GFGCNGPWSEKDMHCHNHCKSIKGYKGGYCAKGGFICKCY
[0053] After codon optimization, the gene sequence is as follows:
[0054] GGT TTT GGT TGT AAC GGT CCA TGG TCT GAA AAG GAT ATG CAT TGT CAT AAC CAT TGT AAG TCT ATT AAG GGT TAC AAG GGT GGT TAC TGT GCT AAG GGT GGT TTT ATT TGT AAG TGT TAC
[0055] 2. Gene expression cassette design
[0056] protected base XhoⅠ Kex 2 MP1106 kill password Xba I protected base
[0057] When designing the gene expression cassette, in order to connect the gene into the pPICZαA vector, two restriction enzyme sites Xho Ⅰ and Xba Ⅰ in the multiple cloning sit...
Embodiment 2
[0064] Embodiment 2 Preparation of recombinant yeast containing the gene of MP1106
[0065] 1. Acquisition and subcloning of genes and expression cassettes
[0066] The plasmid mini-extraction kit extracts the pUC57 plasmid containing MP1106 and the expression cassette (operate according to the kit instructions), and then performs double digestion with Xho I and Xba I endonucleases, and the vector pPICZαA is also carried out with the same enzyme digestion system, the system is as follows :
[0067]
[0068] 37°C, water bath for 4h.
[0069] Then the two obtained double-digested fragments were ligated with T4 DNA ligase, and the ligation system was as follows:
[0070]
[0071] 2. The recombinant plasmid pPICMP1106 was linearized with enzyme Pme Ⅰ and then used for yeast transformation. The linearization system is as follows:
[0072]
[0073]
[0074] Reaction conditions: 37°C, 4h.
[0075] 3. Preparation of competent Pichia pastoris X-33
[0076] Pick a sing...
Embodiment 3
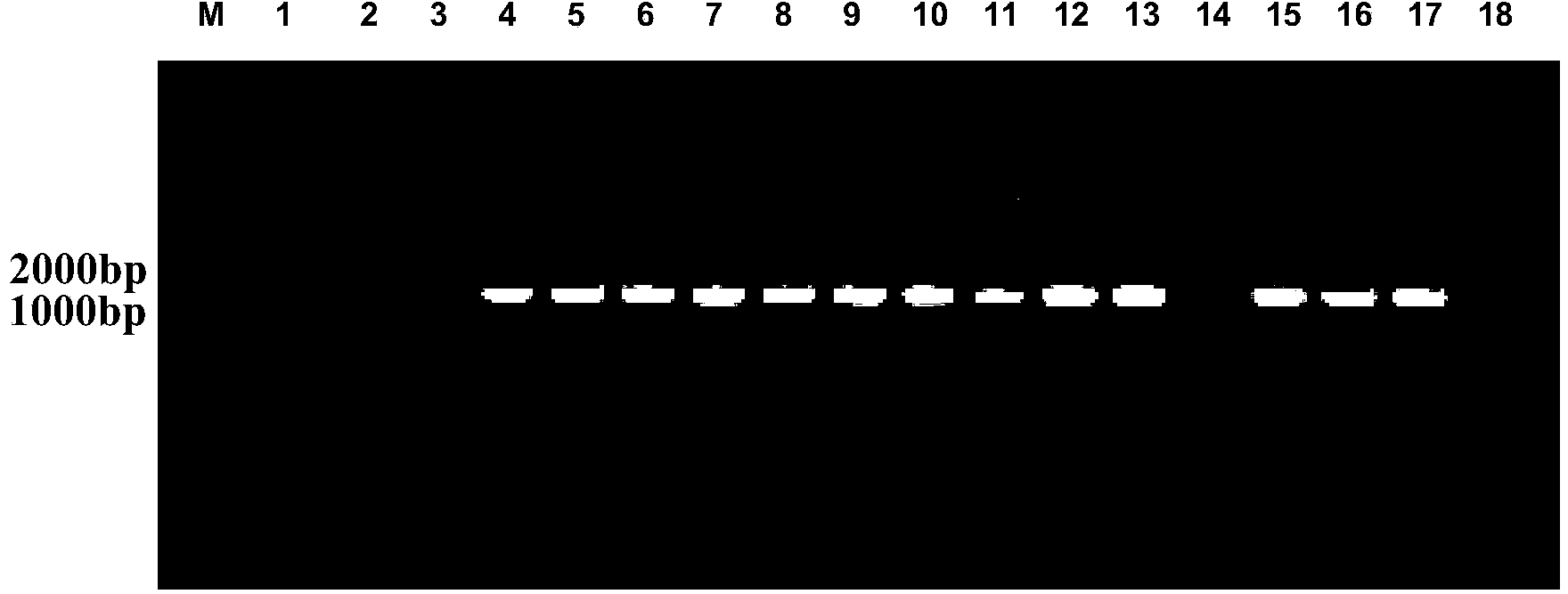
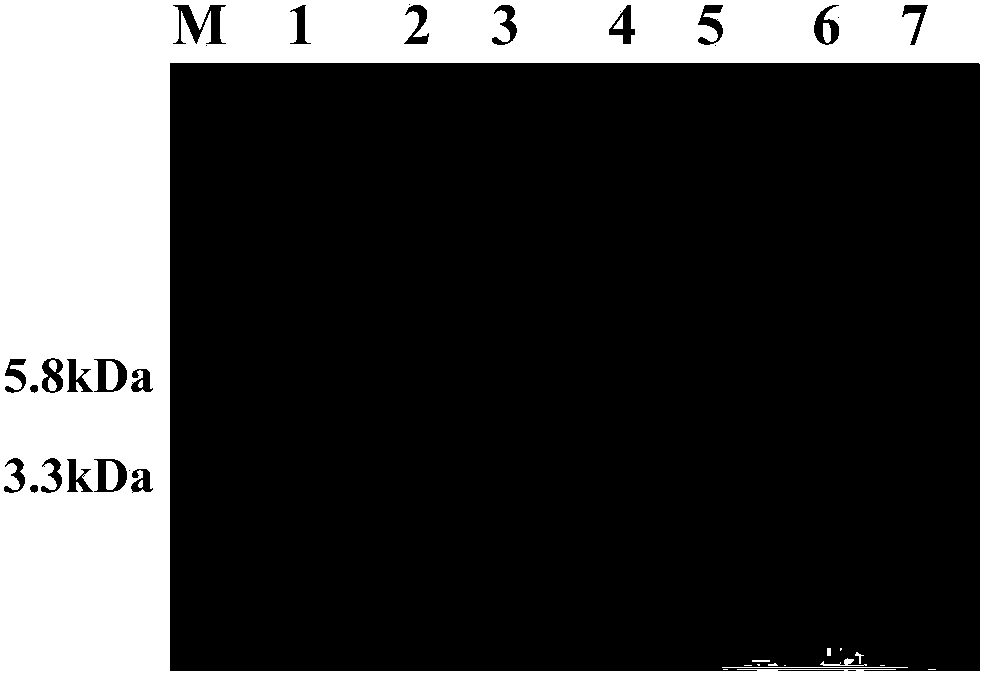
[0086] Example 3 Shake flask horizontal screening of positive transformants and high-density fermentation
[0087] 1. Screening of positive transformants
[0088] Pick the identified positive transformant in 10ml YPD liquid medium, culture at 30°C, 250rpm for 16-18h, inoculate the overnight bacterial solution in 10ml BMGY medium with 1% inoculum, cultivate to OD at 30°C, 250rpm 600nm About 5.0, collect the bacterial liquid, 4°C, 4000rpm, centrifuge for 5 minutes, remove the supernatant, collect the bacterial cells, resuspend the cells with 50ml BMMY medium until the OD600nm is about 1.0, transfer the above bacterial liquid to a 250ml shake flask, bottle Cover the mouth with 4 layers of sterilized gauze to start the induction, which is counted as the initial induction 0h, and then add anhydrous methanol to the final concentration of 0.5% every 24h, induce 120h, and respectively at 0h, 24h, 48h, 72h, 96h, 120h Take 500 μl, centrifuge at 12,000 rpm for 10 minutes, collect the su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com