Method for preparing crystals and application of crystals
A technology of crystal form and crystal seed, applied in the field of crystal preparation and its use, can solve the problems of undocumented preparation method, difficult control of product crystal form, difficult crystal form B, etc., and achieve the effect of repeatable production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of Form B Seed Crystals
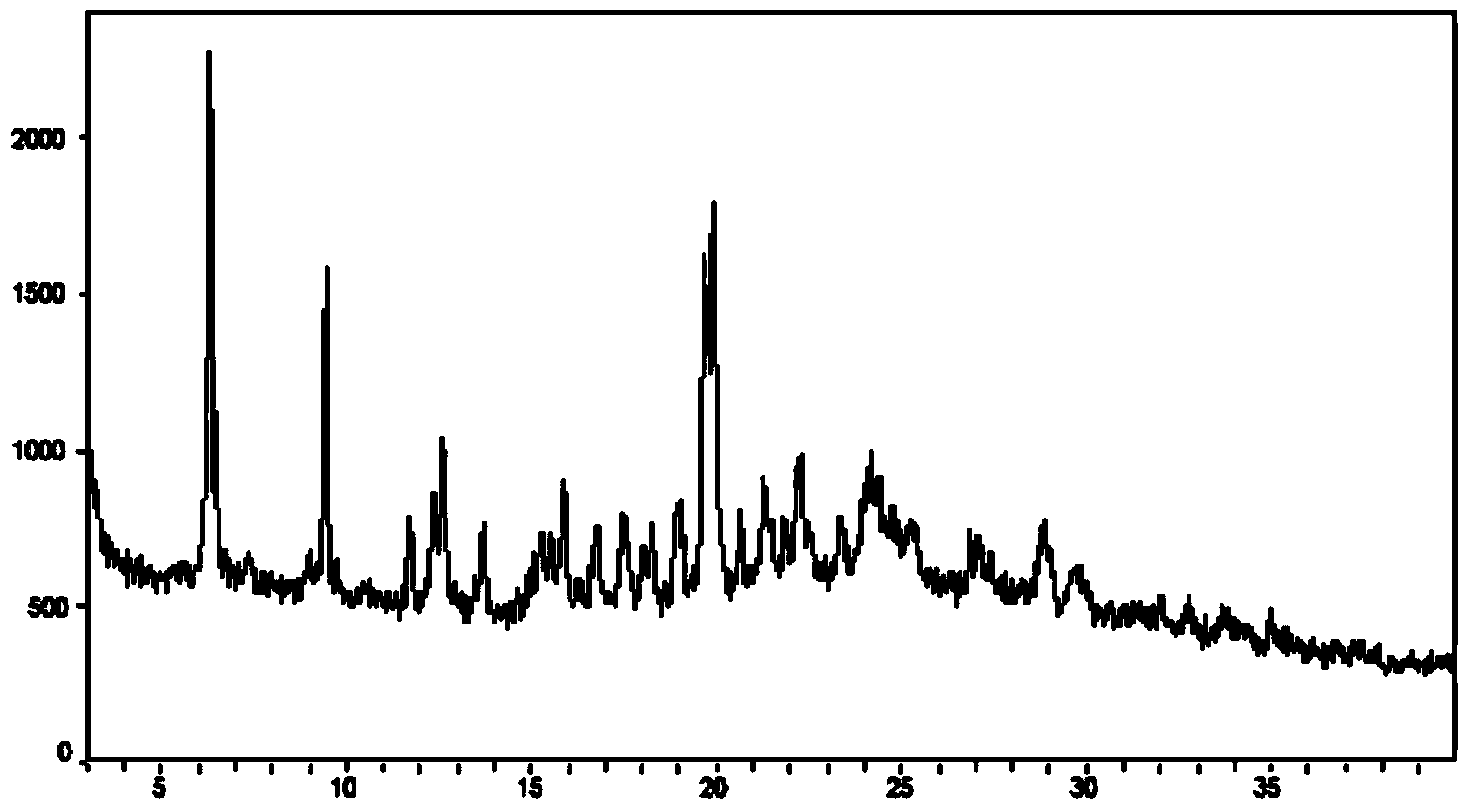
[0055] Suspend 50 mg of Canagliflozin hemihydrate (form A) in 5 mL of water, then heat to 85° C., add 20 mg of mannitol, and stir. The temperature of the above heated solution was lowered to 5 degrees Celsius at a cooling rate of 10 degrees Celsius per hour. During the cooling process, obvious white solids were produced, and these white solids were collected by filtration. Use after natural drying 1 H-NMR characterizes the white solid, and NMR data show that the white solid only contains Canagliflozin and water. NMR data are shown as follows: 1H NMR (d6-DMSO, 400 MHZ): 2.26 (3H, s), 3.13-3.28 (4H, m), 3.44 (1H, m), 3.69 (1H, m), 3.96 (1H, d, J=9.2HZ), 4.10, 4.15(each 1H, d, J=16.0HZ), 4.44(1H, t, J=5.6HZ), 4.73(1H, d, J=6.0HZ), 4.92(2H , d, J=4.8HZ), 6.80(1H, d, J=3.6HZ), 7.11-7.16(2H, m), 7.18-7.25(3H, m), 7.28(1H, d, J=3.6HZ) , 7.59 (2H, dd, J=8.8, 5.4HZ). Then XRPD was used to characterize the sample, and the result showed ...
Embodiment 2
[0062] Preparation of Form B
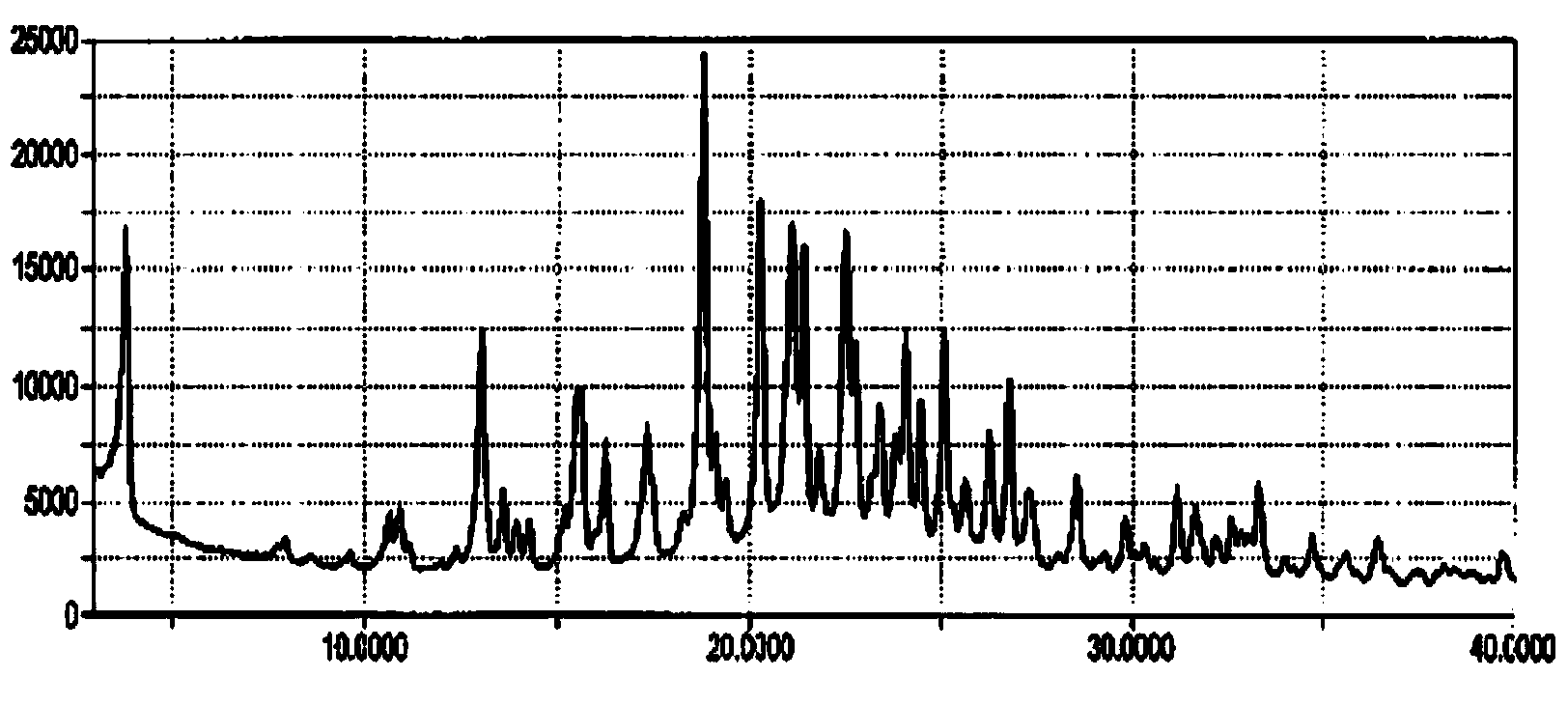
[0063] 156mg of Canagliflozin hemihydrate (crystal form A), suspended in 22mL of water, then heated to 85°C, cooled to 75°C at a rate of 10°C / hour, and added 10mg of crystal form B seed crystals (in Example 1 prepared), continue to cool down to 5°C at a rate of 10°C / hour, and all the above operations are completed under stirring. After cooling down, a white solid was produced, and stirring was continued overnight. The white solid was collected by filtration and dried under vacuum at room temperature for 1 hour. dry solid 1 H-NMR and XRPD analysis and characterization showed that the obtained crystal form was Form B.
Embodiment 3
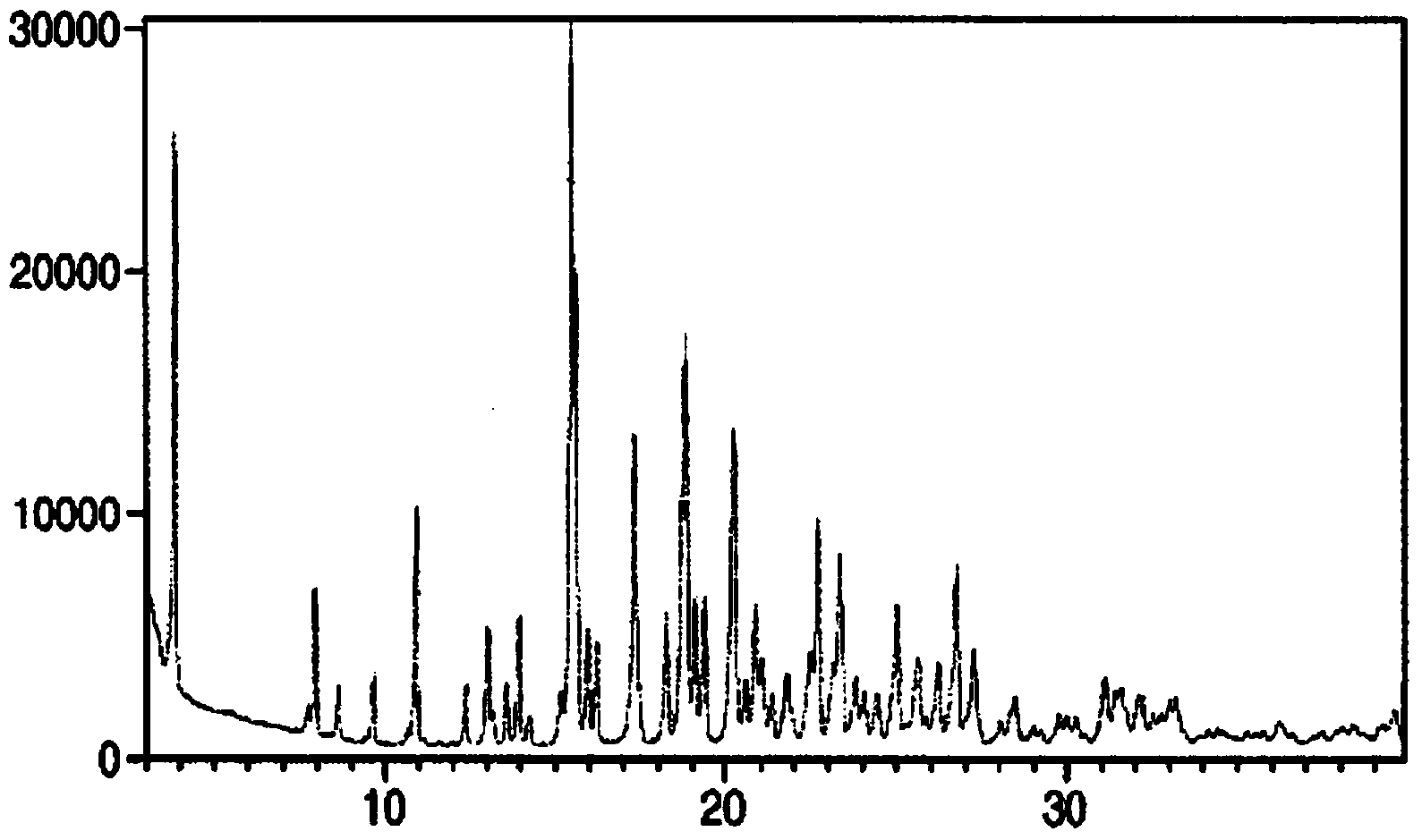
[0065] 200mg of Canagliflozin hemihydrate (crystal form A), suspended in 20mL of water / ethanol mixed solvent (ethanol content 10%, v / v), then heated to 70 ° C, at a rate of 10 ° C / hour, cooled to At 65°C, add 10mg of Form B seed crystals (prepared in Implementation 1), and continue to cool down to 5°C at a rate of 10°C / hour, and all the above operations are completed under stirring. After cooling down, a white solid was produced, and stirring was continued overnight. The white solid was collected by filtration and dried under vacuum at room temperature for 1 hour. dry solid 1 H-NMR and XRPD analysis and characterization showed that the obtained crystal form was Form B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com