Nasal administration preparation containing recombinant human growth hormone and preparation method thereof
A technology of nasal administration preparation and human growth hormone, which is applied in the field of medicine to achieve the effects of increasing nasal retention time, improving bioavailability, and increasing nasal absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
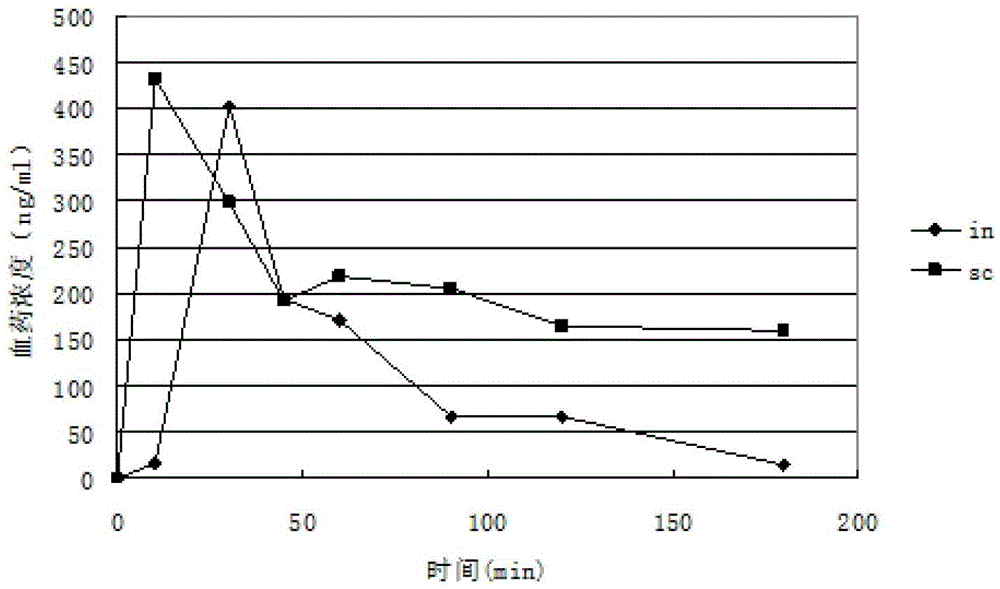
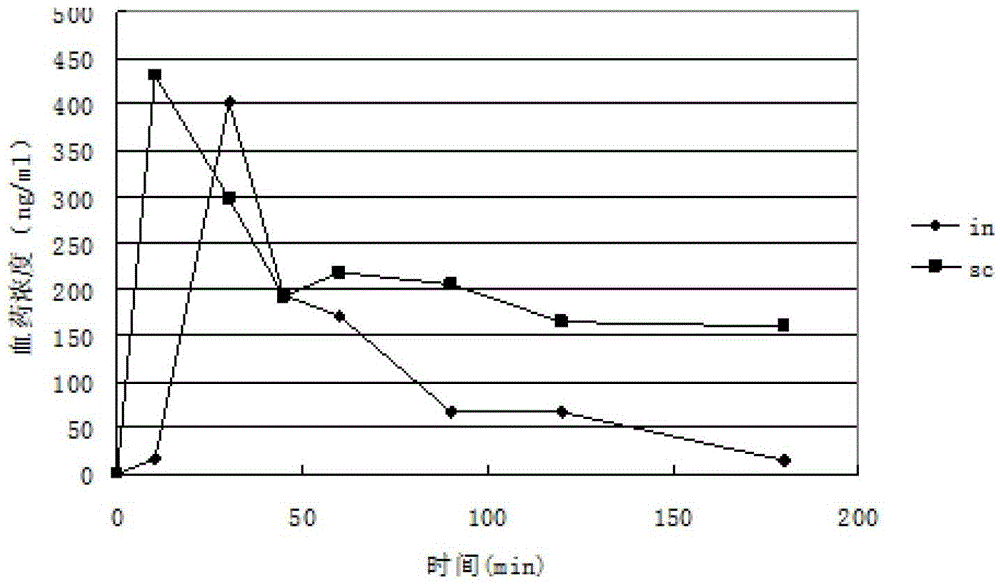
Image
Examples
Embodiment 1
[0043] Recipe: (weight percent)
[0044]
[0045]
[0046] The bioadhesive material chitosan (deacetylation degree greater than 85%) was dissolved in acetic acid-sodium acetate solution to obtain solution A.
[0047] Add recombinant human growth hormone to A solution;
[0048] Add mannitol, lauryl maltoside, and poloxamer F68, mix evenly, freeze-dry, sieve through 100 meshes to obtain 30-150 μm powder, and pack it into a nasal powder mist device to obtain the product.
[0049]Recombinant human growth hormone is a protein with 191 amino acid residues, which is completely consistent with the structure and amino acid sequence of natural human growth hormone.
Embodiment 2
[0051] Recipe: (weight percent)
[0052]
[0053] Recombinant human growth hormone is a protein composed of 192 amino acid residues with a methionine added to the N-terminal.
[0054] The preparation method is the same as in Example 1.
Embodiment 3
[0056] Recipe: (weight percent)
[0057]
[0058] Recombinant human growth hormone is a recombinant human growth hormone with a molecular weight of 20KD, 176 amino acid residues, and 15 amino acid residues at positions 32-46 of natural human growth hormone.
[0059] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com