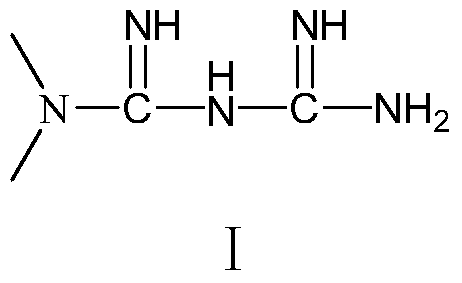
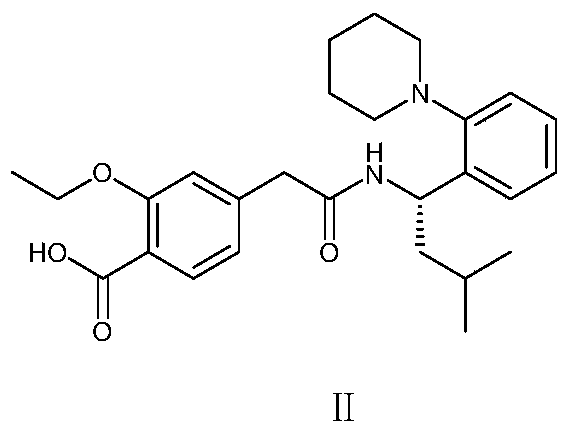
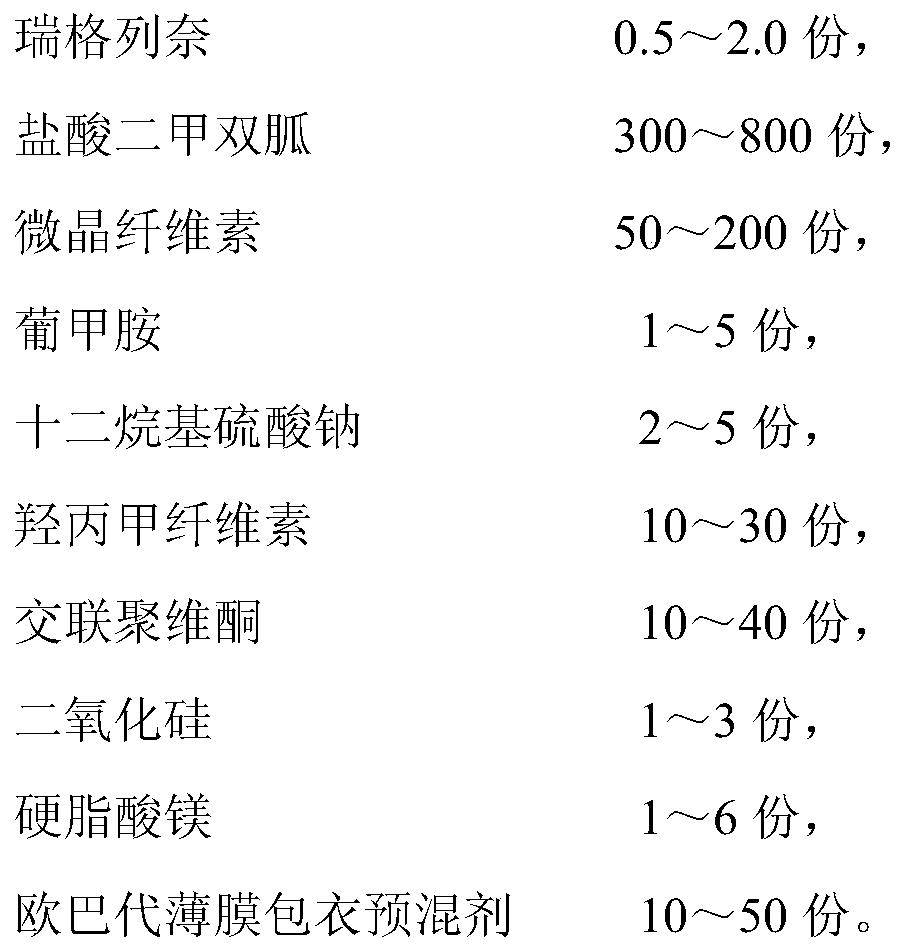
A pharmaceutical composition containing repaglinide metformin and its preparation method
A technology of metformin tablets and metformin hydrochloride, which is applied in the field of pharmaceutical preparations and can solve the problems of metformin not being able to achieve synchronous release, uneven content of repaglinide, and complicated processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Embodiment 1 Preparation of pharmaceutical composition containing repaglinide metformin
[0117] prescription:
[0118]
[0119] The preparation method is prepared according to the following steps:
[0120] 1) Processing of raw and auxiliary materials:
[0121] Micronize repaglinide bulk drug so that d(0.9)≤10 μm, and set aside;
[0122] Metformin hydrochloride 100 mesh sieves are pulverized for subsequent use;
[0123] Crospovidone, meglumine, and sodium lauryl sulfate are passed through a 60-mesh sieve, and set aside;
[0124] 2) Premix:
[0125] a. Take about 2 / 3 of the amount of microcrystalline cellulose, 1 / 2 of the amount of hypromellose, about 2 / 3 of the amount of crospovidone, silicon dioxide and about 1 / 2 of the amount The metformin hydrochloride is put into a multidirectional motion mixer and mixed for 5 to 10 minutes, then the remaining metformin hydrochloride is added to the mixer and mixed for 5 to 10 minutes, dispersed through a 50-mesh sieve to ob...
Embodiment 2
[0138] Embodiment 2 Preparation of pharmaceutical composition containing repaglinide metformin
[0139] prescription:
[0140]
[0141] The preparation method is prepared according to the following steps:
[0142] 1) Processing of raw and auxiliary materials:
[0143] Micronize repaglinide bulk drug so that d(0.9)≤10 μm, and set aside;
[0144] Metformin hydrochloride 120 mesh sieves are pulverized for subsequent use;
[0145] Crospovidone, meglumine, and sodium lauryl sulfate are passed through a 60-mesh sieve, and set aside;
[0146] 2) Premix:
[0147] a. Take half of the amount of microcrystalline cellulose, half of the amount of hypromellose, 1 / 3 of the amount of crospovidone, silicon dioxide and 1 / 4 of the amount of metformin hydrochloride, and put them into a multi-directional motion mixer Mix for 5-10 minutes, then add the remaining metformin hydrochloride into the mixer and mix for 5-10 minutes, disperse with a 30-mesh sieve to obtain the mixture ①, set aside;...
Embodiment 3
[0160] Embodiment 3 Preparation of pharmaceutical composition containing repaglinide metformin
[0161] prescription:
[0162]
[0163]
[0164] The preparation method is prepared according to the following steps:
[0165] 1) Processing of raw and auxiliary materials:
[0166] Micronize repaglinide bulk drug so that d(0.9)≤10 μm, and set aside;
[0167] Metformin hydrochloride 120 mesh sieves are pulverized for subsequent use;
[0168] Crospovidone, meglumine, and sodium lauryl sulfate are passed through a 60-mesh sieve, and set aside;
[0169] 2) Premix:
[0170]a. Take about two-thirds of microcrystalline cellulose, two-thirds of hypromellose, one-half of crospovidone, silicon dioxide and about one-third of Metformin hydrochloride is put into a multidirectional motion mixer and mixed for 5 to 10 minutes, then the remaining metformin hydrochloride is added to the mixer and mixed for 5 to 10 minutes, dispersed through a 30-mesh sieve to obtain the mixture ①, and set...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com