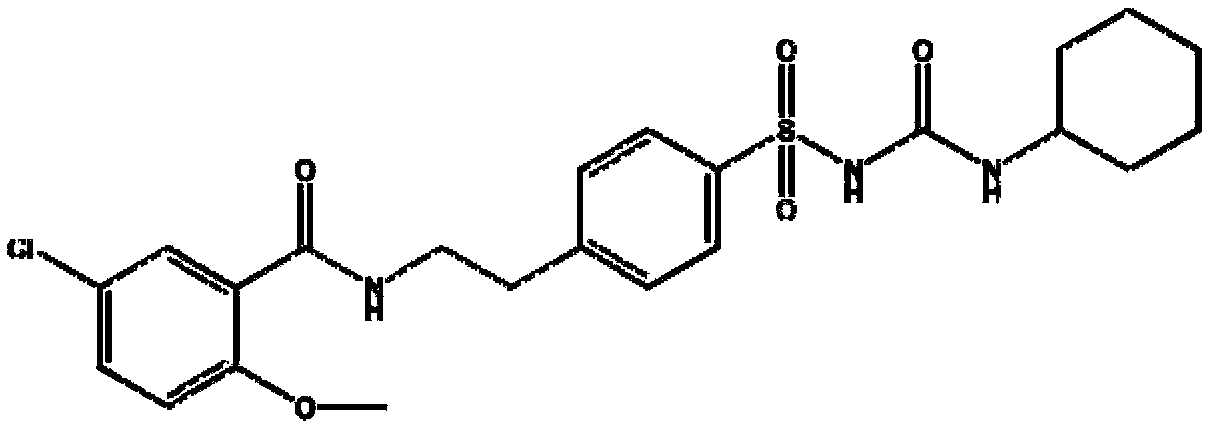
Glibenclamide tablet and preparation method thereof
A technology of glyburide and urea tablets, applied in the directions of sulfonylurea active ingredients, pill delivery, metabolic diseases, etc., to reduce the cost of excipients, improve the stability and quality controllability, and improve the dissolution rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
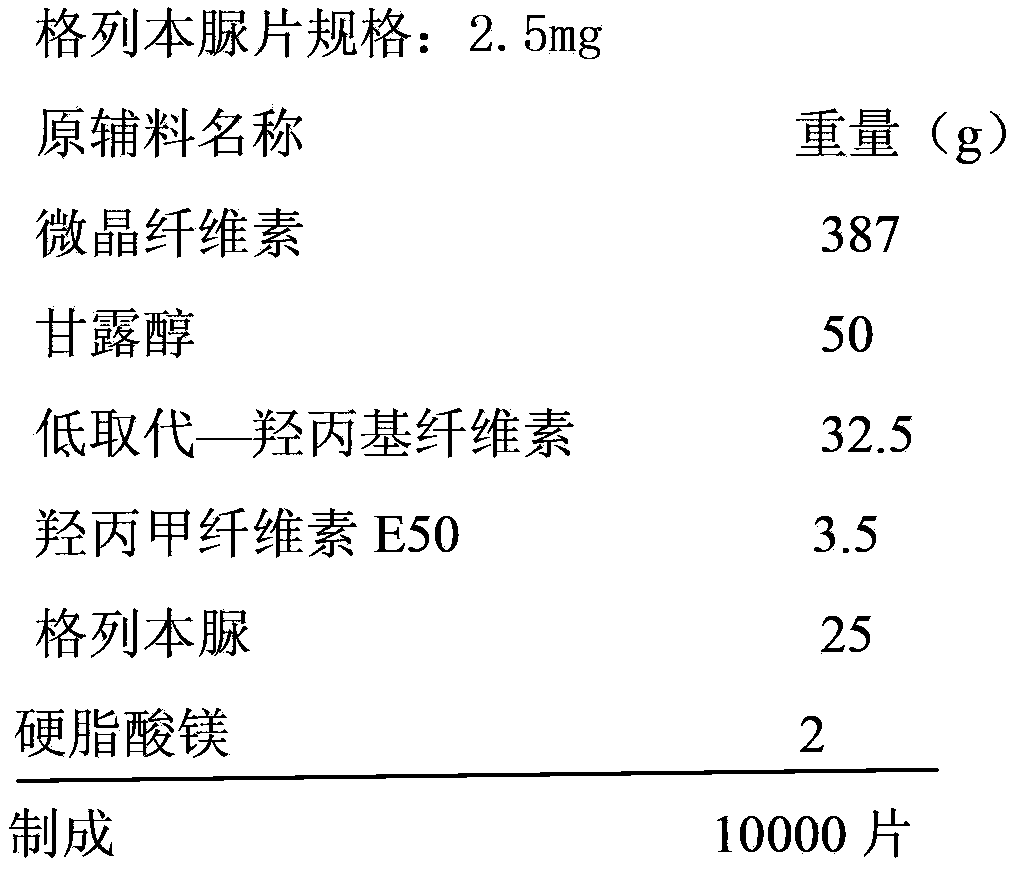
[0034]
[0035] Preparation method and steps:
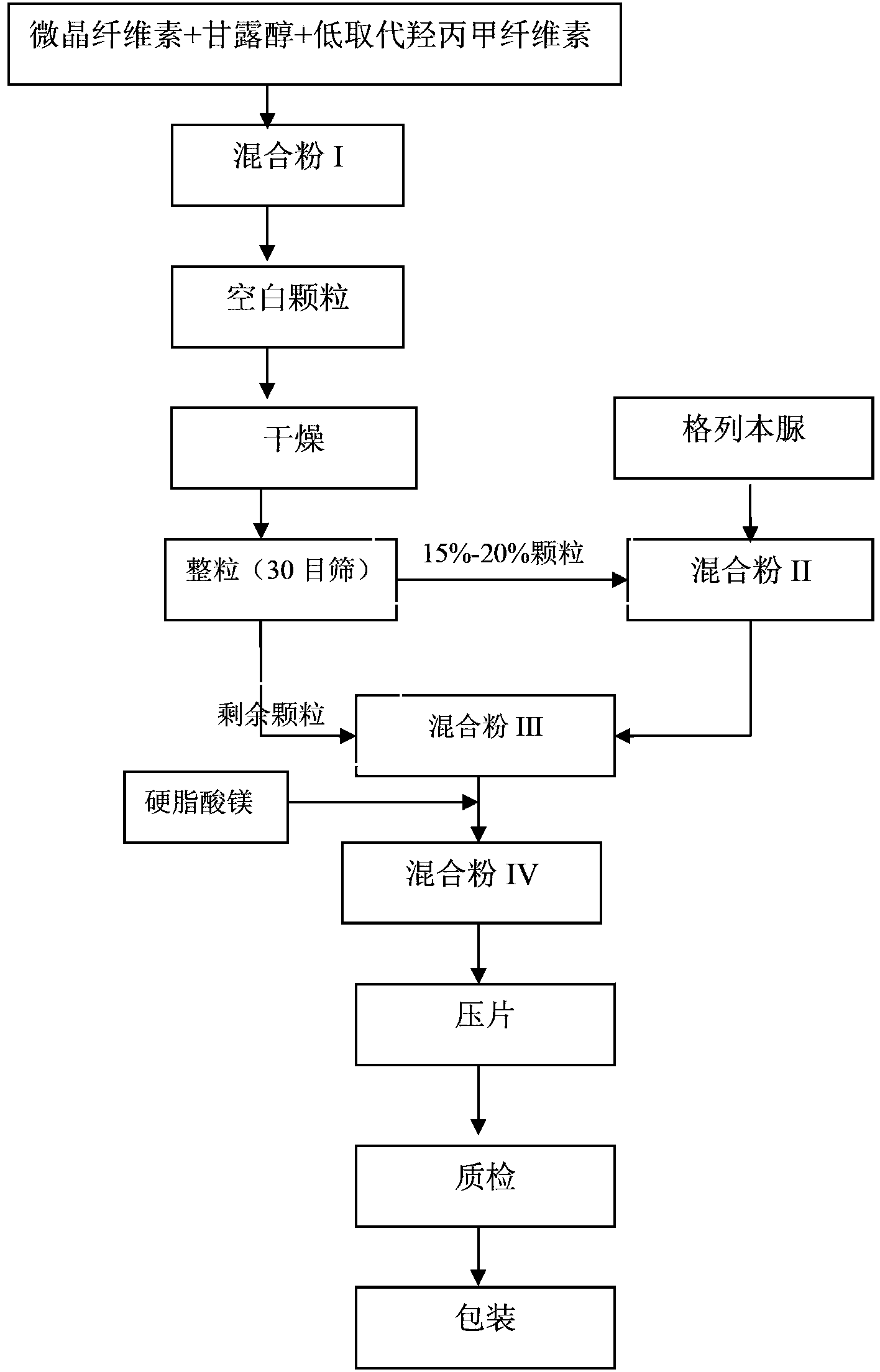
[0036] 1) Mix the above weight of microcrystalline cellulose, mannitol and low-substituted hydroxypropyl cellulose to obtain mixed powder I; add mixed powder I to a high-efficiency wet granulator, and stir at high speed for 3 minutes; 2) Add 175g2 % hypromellose E50 aqueous solution into the mixed powder I to make fine sand-like blank granules; 2% hypromellose E50 aqueous solution is prepared by hypromellose E50 and water in a weight ratio of 2:98 3) Move the blank granules into an oven, ventilate and dry at 70°C for 2 hours, so that the moisture content in the blank granules is controlled at 2.2-3.0%; after drying, pass the blank granules through a 20-mesh sieve, and pass through The blank granule of 50 mesh sieve is not less than 80%; 4) 25g of glibenclamide is fully mixed with 15% of the blank granule according to the equal amount incremental method to obtain the mixed powder II; in this embodiment, the equal amount increme...
Embodiment 2
[0038]
[0039] Preparation method and steps:
[0040]1) Mix the above weight of microcrystalline cellulose, mannitol and low-substituted hydroxypropyl cellulose to obtain mixed powder I; add mixed powder I to a high-efficiency wet granulator, and stir at high speed for 5 minutes; 2) Add 750g2 % hypromellose E50 aqueous solution into the mixed powder I to make fine sand-like blank granules; 2% hypromellose E50 aqueous solution is prepared by hypromellose E50 and water in a weight ratio of 2:98 3) Move the blank granules into an oven, ventilate and dry at 73°C for 2 hours, so that the moisture content in the blank granules is controlled at 2.2-3.0%; after drying, pass the blank granules through a 30-mesh sieve, and pass through The blank granule of 50 mesh sieve is not less than 80%; 4) 25g of glibenclamide is fully mixed with 18% of the blank granule according to the equal amount incremental method to obtain the mixed powder II; in this embodiment, the equal amount incremen...
Embodiment 3
[0042]
[0043] Preparation method and steps:
[0044] 1) Mix the above weight of microcrystalline cellulose, mannitol and low-substituted hydroxypropyl cellulose to obtain mixed powder I; add mixed powder I to a high-efficiency wet granulator, and stir at high speed for 7 minutes; 2) Add 700g2 % hypromellose E50 aqueous solution into the mixed powder I to make fine sand-like blank granules; 2% hypromellose E50 aqueous solution is prepared by hypromellose E50 and water in a weight ratio of 2:98 3) Move the blank granules into an oven, ventilate and dry at 75°C for 2 hours, so that the moisture content in the blank granules is controlled at 2.2-3.0%; after drying, pass the blank granules through a 40-mesh sieve, and pass through The blank granule of 50 mesh sieve is not less than 80%; 4) 25g of glibenclamide is fully mixed with 20% of the blank granule according to the equal amount incremental method to obtain the mixed powder II; in this embodiment, the equal amount increme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com