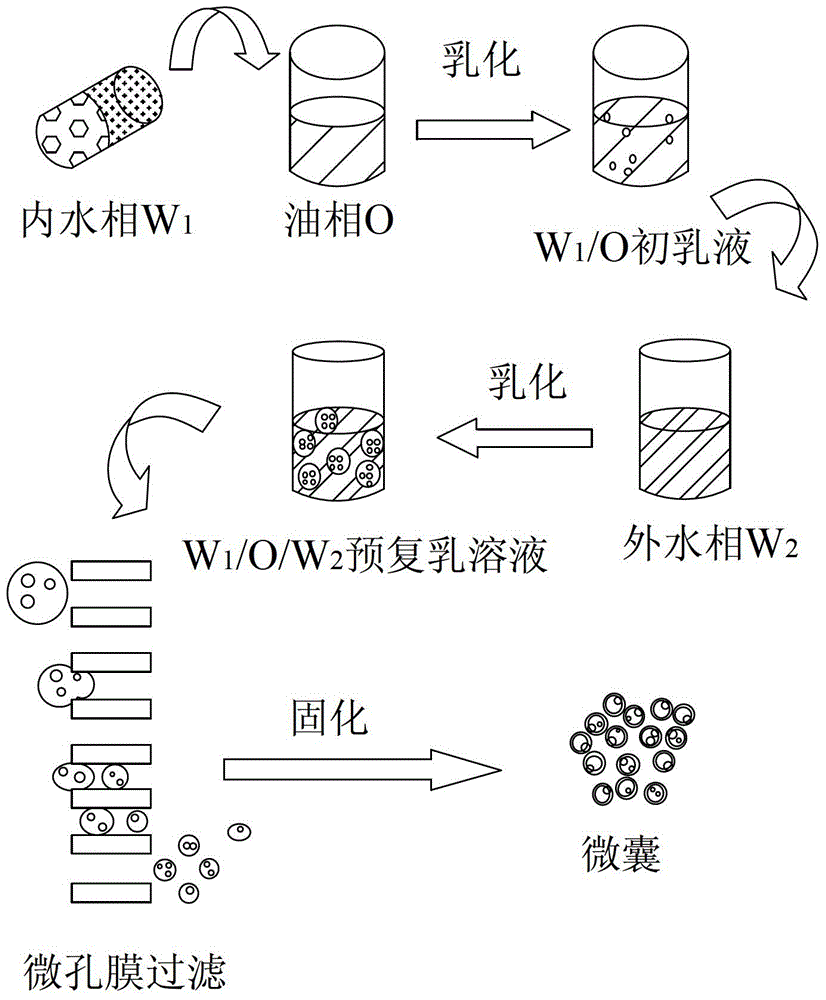
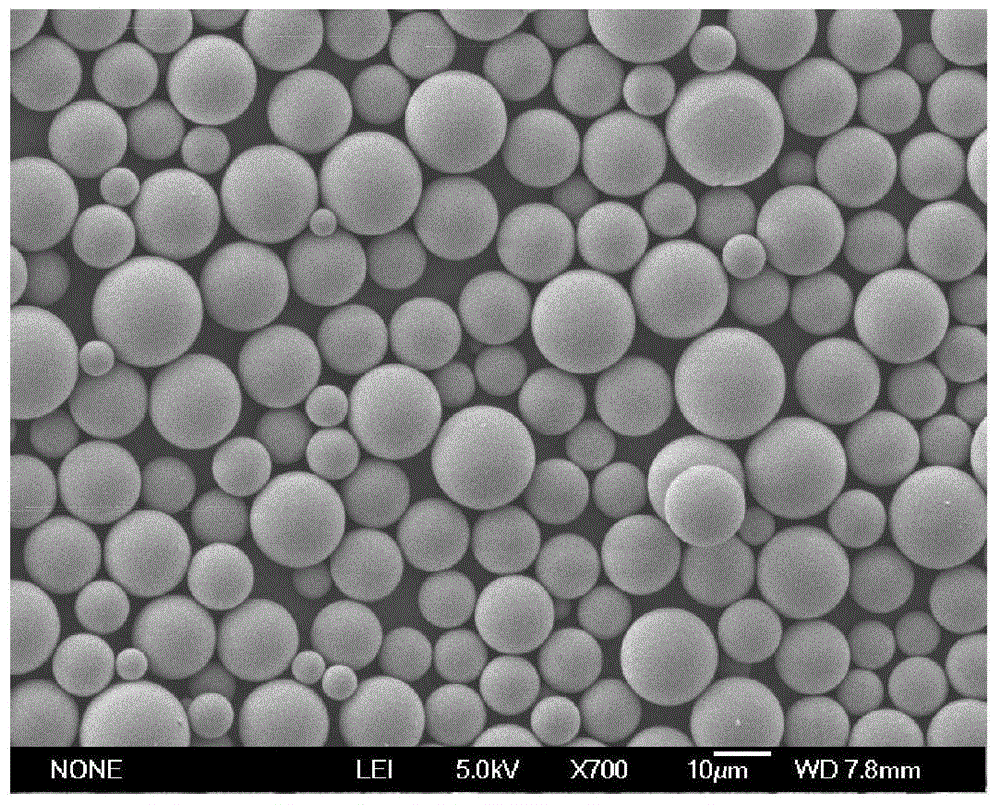
A kind of microcapsules for encapsulation of small molecule hydrophilic drug sustained release and preparation method thereof
A technology of hydrophilic drugs and small molecules, which is applied in the field of medicine, can solve the problems of low burst release rate, low drug loading, and high burst release rate, and achieve the effects of reducing drug burst release, increasing embedding rate, and reducing diffusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] A preparation method of microcapsules with embedded small-molecule hydrophilic drugs, including the following steps:
[0077] (1) Dissolve 300 mg of gelatin in 1 mL of water, and then dissolve 40 mg of triptorelin acetate in the above aqueous gelatin solution as the internal water phase W 1 ; Dissolve 0.1 g of polylactic acid-glycolic acid copolymer (PLGA) with a molecular weight of 60,000 in 10 mL of dichloromethane (MC) as the oil phase O; take 50 mL of 0.1wt% polyvinyl alcohol (PVA) aqueous solution as Outer water phase W 2 ; Place a hydrophilic membrane with a pore size of 50.2μm in water to soak the pore membrane fully;
[0078] (2) The internal water phase W 1 Add to the oil phase O, homogenize and emulsify for 30s to obtain W 1 / O type primary emulsion; among them, the internal water phase W 1 The volume ratio to oil phase O is 1:10;
[0079] (3) Put W 1 / O type primary emulsion is added to the outer water phase W 2 Medium, magnetic stirring 300rpm stirring 1min to prep...
Embodiment 2
[0095] A preparation method of microcapsules with embedded small-molecule hydrophilic drugs, including the following steps:
[0096] (1) Dissolve 20 mg of starch in 1 mL of water, and then dissolve 1 mg of recombinant human growth hormone (molecular weight 22kDa) in the above aqueous starch solution as the internal water phase W 1 , Dissolve 1g of a mixture of polylactic acid with a molecular weight of 10,000 and a polylactic acid-polyglycolic acid copolymer with a molecular weight of 10,000 (mass ratio 1:1) in 5mL of carbon disulfide as the oil phase O; take 50mL of 0.1wt % Of polyglycerol fatty acid ester aqueous solution is the outer water phase W 2 ; Place a hydrophilic membrane with a pore size of 0.5μm in water to soak the pore membrane fully;
[0097] (2) The internal water phase W 1 Add to the oil phase O, homogenize and emulsify for 30s to obtain W 1 / O type primary emulsion; among them, the internal water phase W 1 The volume ratio with oil phase O is 1:5;
[0098] (3) Put ...
Embodiment 3
[0107] A preparation method of microcapsules with embedded small-molecule hydrophilic drugs, including the following steps:
[0108] (1) Dissolve 200 mg of a mixture of gum arabic and gelatin (mass ratio 5:1) in 1 mL of water, and then dissolve 50 mg of adriamycin (molecular weight 543Da) in the above gum arabic aqueous solution as the internal water phase. 1 ; 0.1g of polyorthoester with a molecular weight of 40,000 was dissolved in 1mL of chloroform as the oil phase O; 4mL of 10wt% polyoxyethylene sorbitan monooleate aqueous solution was used as the outer water phase W 2 ; Place a hydrophilic membrane with a pore size of 200μm in water so that the pore membrane is fully wetted;
[0109] (2) The internal water phase W 1 Add to the oil phase O, homogenize and emulsify for 30s to obtain W 1 / O type primary emulsion; among them, the internal water phase W 1 The volume ratio to oil phase O is 1:1;
[0110] (3) Put W 1 / O type primary emulsion is added to the outer water phase W 2 Medium,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com