Dezocine freeze-dried powder injection and preparation method thereof
A technology of freeze-dried powder injection and dezocine, which is applied in the field of pharmaceuticals, can solve the problems of increased burden on preparation quality control and clinical use risks, and achieve the effect of reducing additional risks and avoiding potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
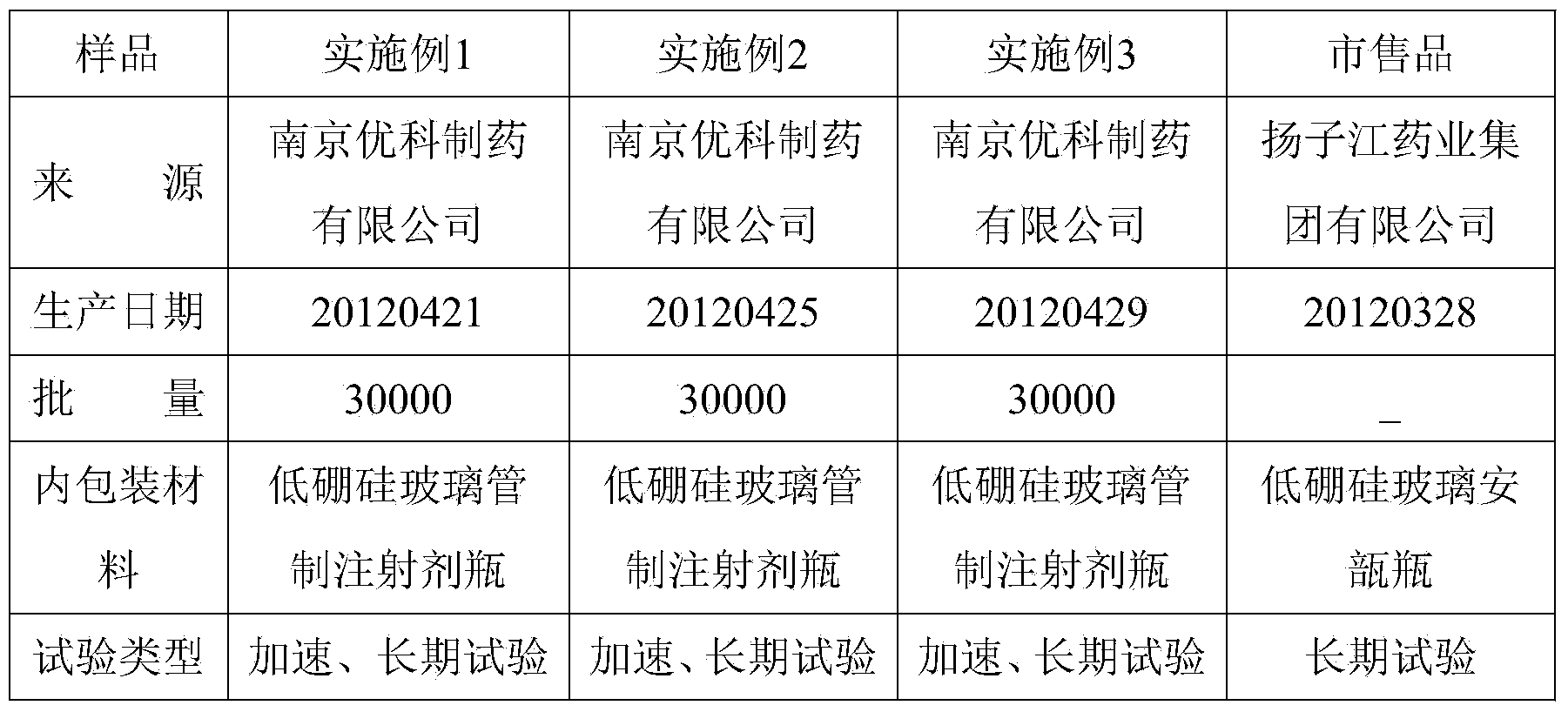
Embodiment 1
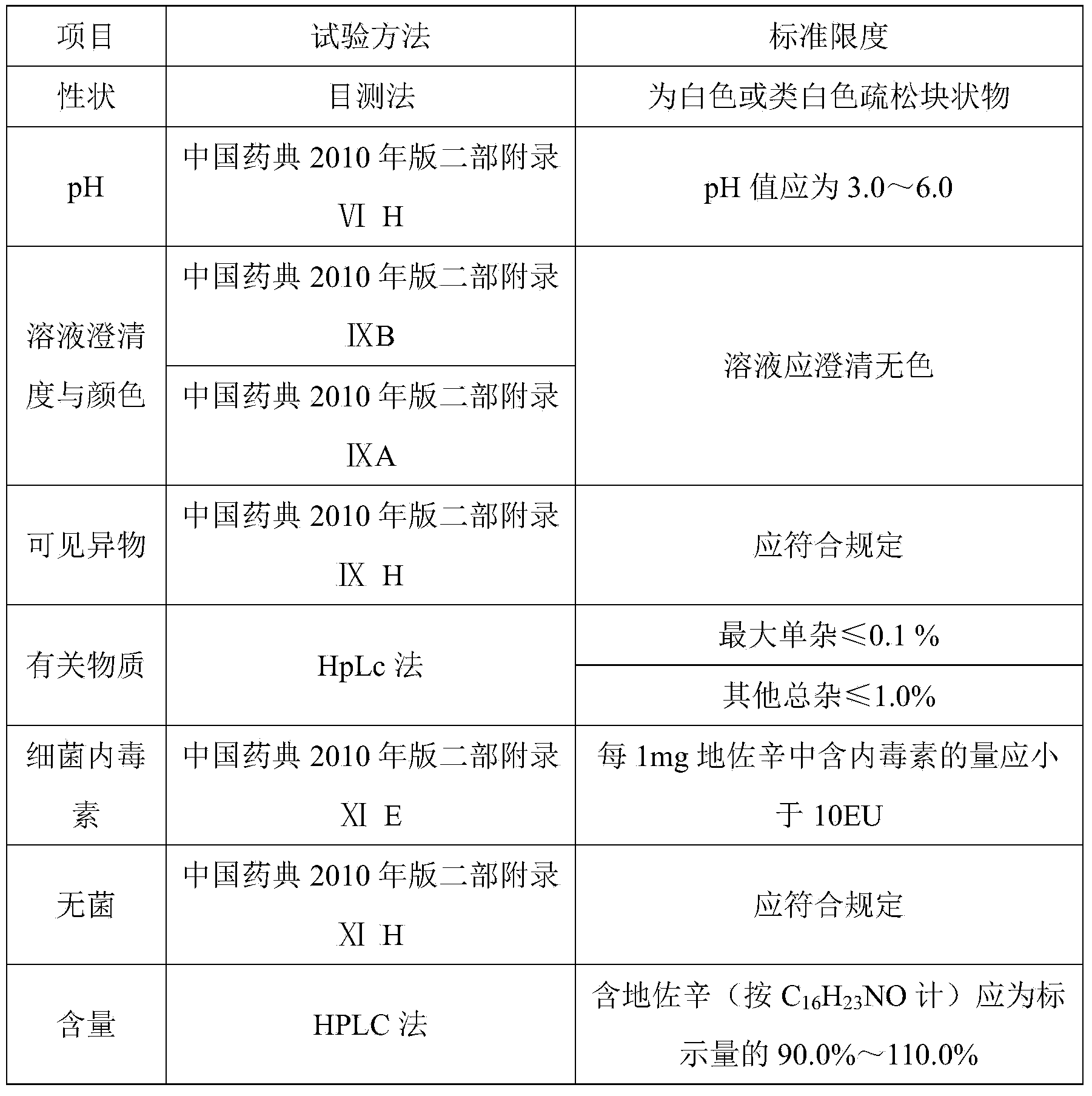
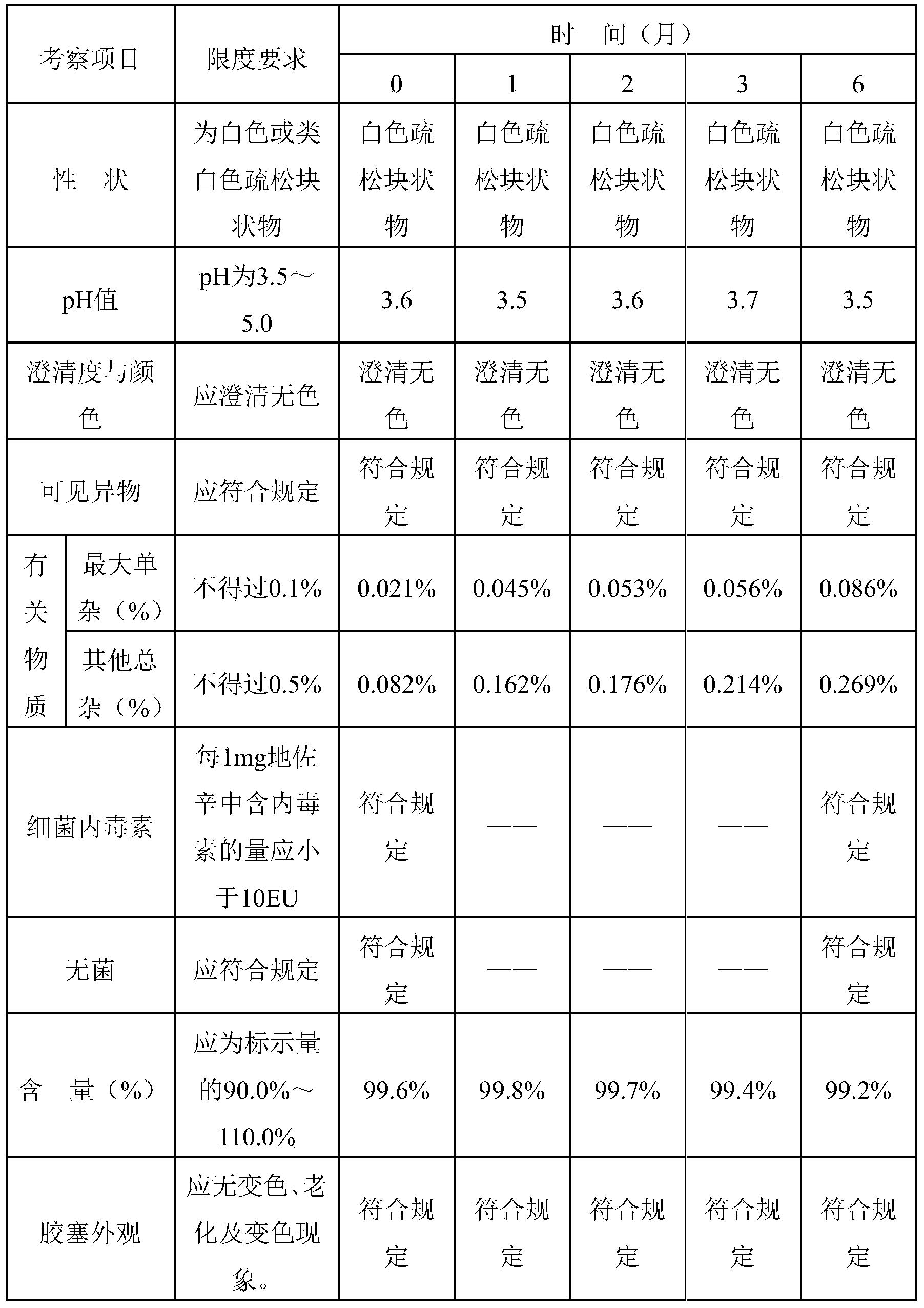
[0036] Prepare 1000 tubes of 2 mL dezocine for injection. The prescription is: 5 g of dezocine, 0.5 g of edetate disodium, 15.0 g of sodium chloride, 10.0 g of hydrochloric acid, sodium carbonate to adjust the pH value to 3.0-6.0, and add water for injection to 2000 mL .
[0037] The preparation method is as follows: 1) After mixing water for injection and hydrochloric acid, adding prescribed amount of dezocine to make it dissolve. 2) Add other excipients to dissolve according to the prescription, adjust the appropriate pH value to 3.0-6.0 with sodium carbonate, add charcoal for adsorption, filter to remove charcoal and sterilize. 3) Under aseptic operation, subpackage and freeze-dry.
Embodiment 2
[0039] Prepare 1000 vials of 5 mL dezocine for injection. The prescription is: 10 g of dezocine, 480 g of glycine, 2 g of calcium sodium edetate, 60 g of lactic acid, sodium hydroxide to adjust the pH to 3.0-6.0, and water for injection to 5000 mL.
[0040] Preparation method: 1) Weigh water for injection and mix with lactic acid, add dezocine to dissolve it. 2) According to the prescription, add other excipients to dissolve, adjust the pH value to 3.0-6.0 with sodium hydroxide solution, add charcoal for adsorption, and filter to remove charcoal and bacteria. 3) Under aseptic operation, subpackage and freeze-dry.
Embodiment 3
[0042] Prepare 1000 vials of 2 mL dezocine for injection. The prescription is: 5 g of dezocine, 10 g of citric acid, sodium hydroxide, appropriate amount to adjust the pH to 3.0-6.0, 0.2 g of sodium ascorbate, 100 g of mannitol, and water for injection to 2000 mL.
[0043] Preparation method: 1) After dissolving in water for injection and citric acid, weigh the prescribed amount of dezocine to dissolve it. 2) Add other excipients to dissolve according to the prescription, adjust the pH to 3.0-6.0 with appropriate amount of sodium hydroxide, add charcoal for adsorption, filter to remove charcoal and sterilize. 3) Under aseptic operation, subpackage and freeze-dry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com