Self-activation vanadate fluorescent powder, preparation method and applications thereof
A type of vanadate and phosphor technology, which is applied in the field of self-activating vanadate phosphor and its preparation, can solve problems such as low photoelectric conversion efficiency, and achieve the effects of high repeatability and simple synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
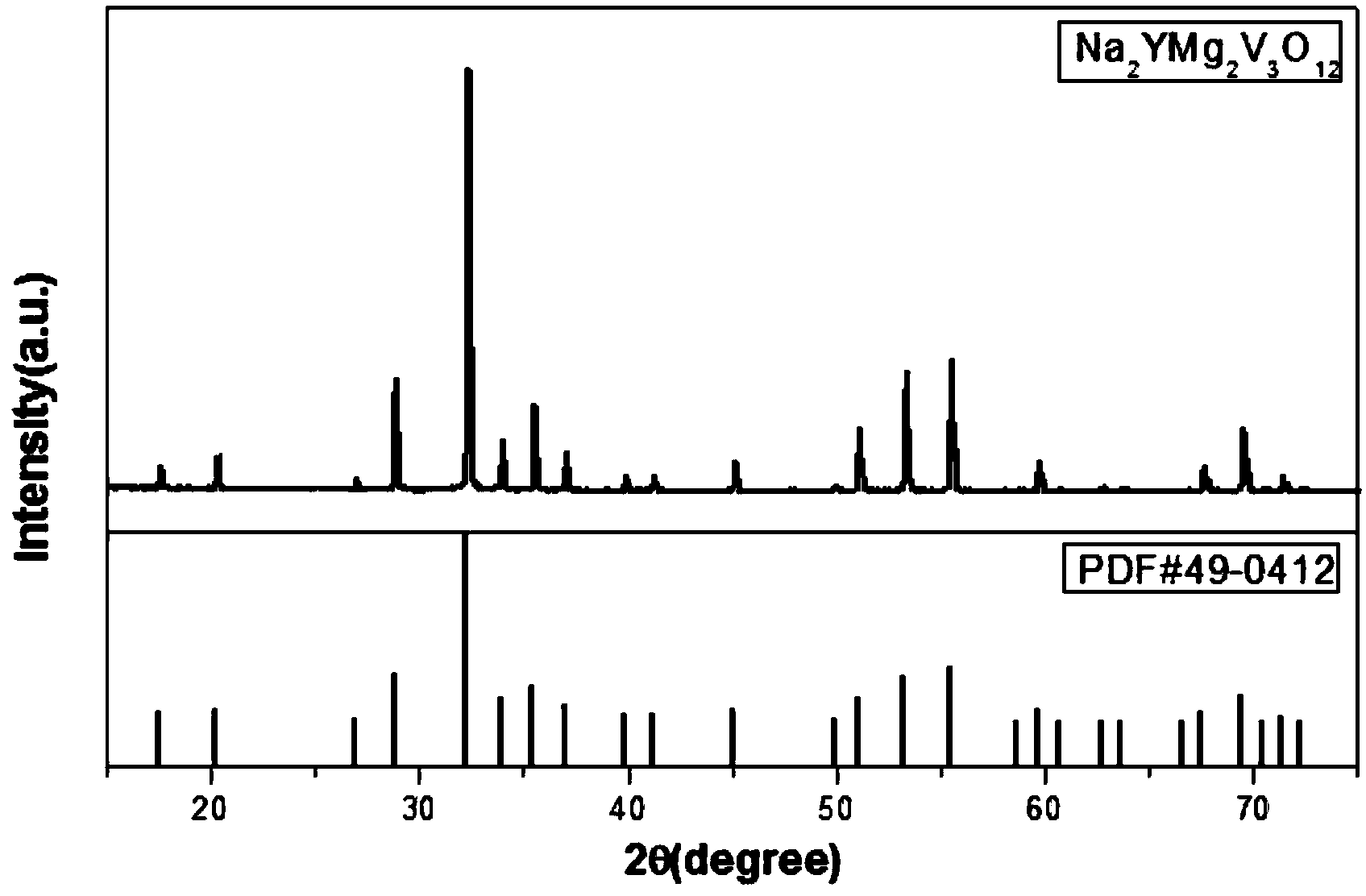
[0032] This embodiment prepares self-activated vanadate Na by sol-gel method 2 YMg 2 V 3 o 12 The sample preparation process is as follows:
[0033] 1) According to the reactant cation molar ratio Na + :Y 3+ :Mg 2+ = 2:1:2 Weigh the raw material Na respectively 2 CO3 (AR)0.2120g, Y(NO 3 ) 3 ·6H 2 O0.7660g, Mg(NO 3 ) 2 ·6H 2 O(AR) 1.0256g, add distilled water and stir to obtain solution A;
[0034] Weigh citric acid 4.2028g and NH 4 VO 3 (AR) 0.7019g, slowly add solution A, stir while adding, until complete reaction, obtain uniform solution A;
[0035] 2) Put the solution into an oven and keep it warm at 60°C for 72 hours to form a uniform transparent colloid, then raise the temperature to 120°C and keep it warm for 12 hours to obtain a brown precursor;
[0036] 3) Fully grind the precursor, pre-calcine at 500°C for 4 hours to obtain a fluffy gray starting material, fully grind the starting material and calcinate at 700°C for 5 hours, cool and grind to obtain a ...
Embodiment 2
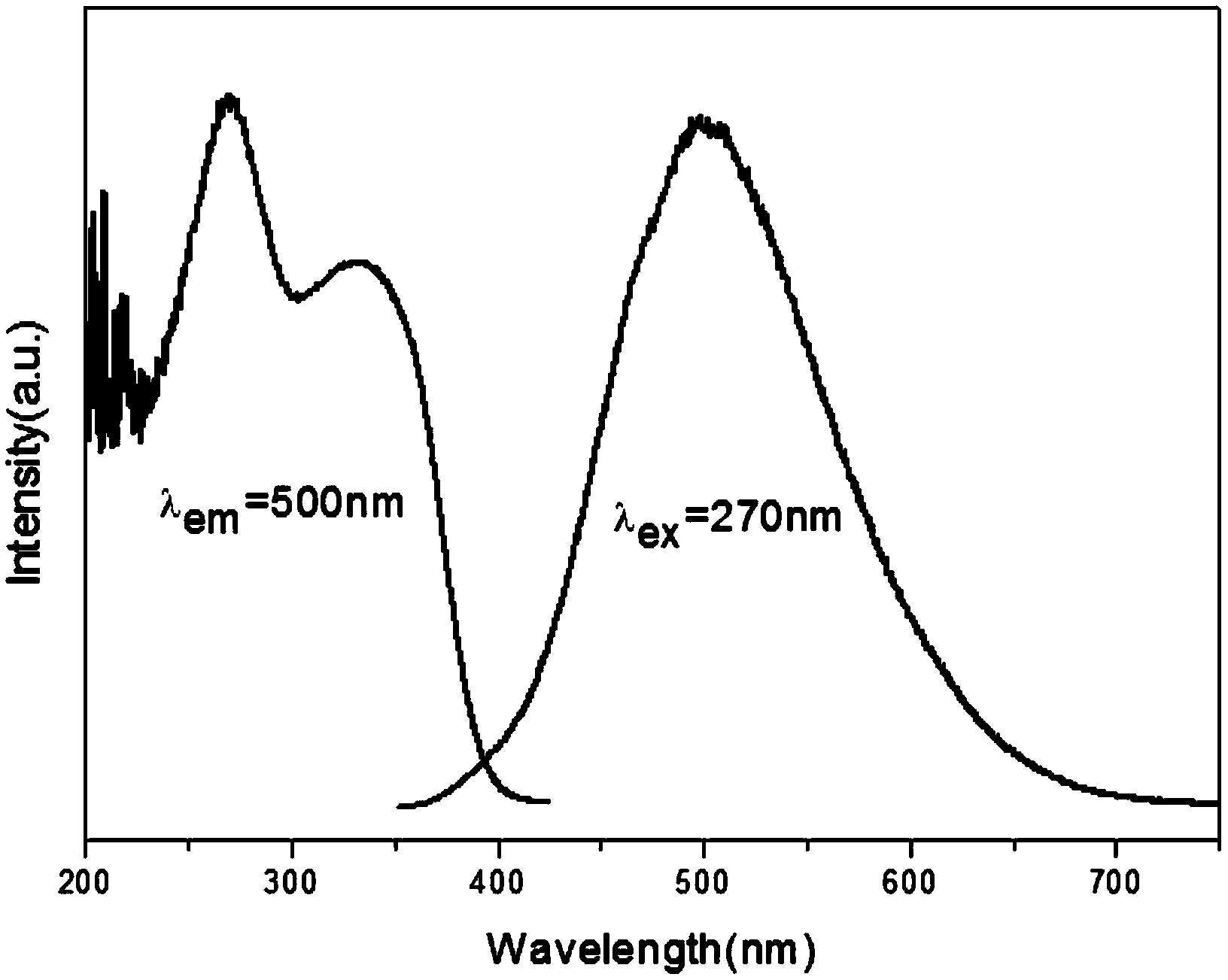
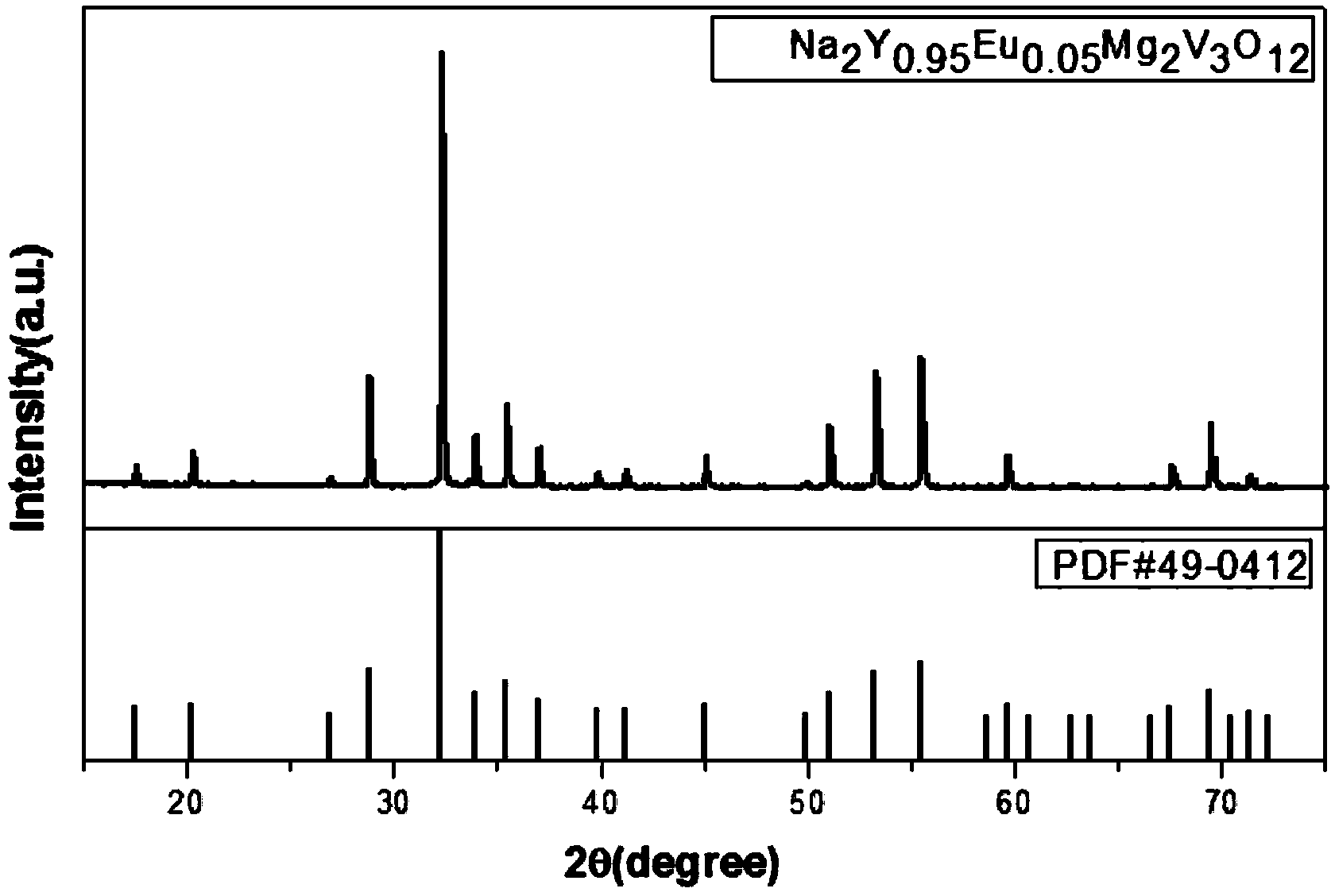
[0039] This embodiment prepares self-activated vanadate Na by sol-gel method 2 Y 0.95 Eu 0.05 Mg 2 V 3 o 12 The sample preparation process is as follows:
[0040] 1) According to the reactant cation molar ratio Na + :Y 3+ :Mg 2+ =2:0.95:2 Weigh the raw material Na respectively 2 CO 3 (AR)0.2120g, Y(NO 3 ) 3 ·6H 2 O0.7277g, Mg(NO 3 ) 2 ·6H 2 O(AR) 1.0256g, measure 0.02mol / L of Eu(NO 3 ) 3 Solution 5mL was mixed and stirred to obtain solution A;
[0041] Weigh citric acid 4.2028g and NH 4 VO 3 (AR) 0.7019g, slowly add solution A, stir while adding, until complete reaction, obtain uniform solution B;
[0042] 2) Put solution B in an oven and keep it warm at 70°C for 48 hours to form a uniform transparent colloid, then raise the temperature to 110°C and keep it warm for 36 hours to obtain a brown precursor;
[0043] 3) Fully grind the precursor, pre-calcine at 500°C for 5 hours to obtain a fluffy gray starting material, fully grind the starting material, calci...
Embodiment 3
[0046] This embodiment prepares self-activated vanadate Na by sol-gel method 2 Y 0.9 Eu 0.1 Mg 2 V 3 o 12 The sample preparation process is as follows:
[0047] 1) According to the reactant cation molar ratio Na + :Y 3+ :Mg 2+ =2:0.9:2 Weigh the raw material Na respectively 2 CO 3 (AR)0.2120g, Y(NO 3 ) 3 ·6H 2 O0.6894g, Mg(NO 3 ) 2 ·6H 2 O(AR) 1.0256g, measure 0.02mol / L of Eu(NO 3 ) 3 Solution 10mL was mixed and stirred to obtain solution A;
[0048] Weigh citric acid 4.2028g and NH 4 VO 3 (AR) 0.7019g, slowly add solution A, stir while adding, until complete reaction, obtain uniform solution B;
[0049] 2) Put solution B in an oven and keep it warm at 70°C for 48 hours to form a uniform transparent colloid, then raise the temperature to 110°C and keep it warm for 36 hours to obtain a brown precursor;
[0050] 3) Fully grind the precursor, pre-calcine at 500°C for 5 hours to obtain a fluffy gray starting material, fully grind the starting material, calcina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com