Preparation method and novel medicinal application of humulus scandens flavone composition
A Humulus flavonoids and composition technology, which is applied in the preparation of Humulus flavonoids composition and the field of new medical applications, can solve problems such as undiscovered, and achieve the effect of clear ingredients and high content of flavonoids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0068] Example 1 Preparation method of humuli flavonoid composition
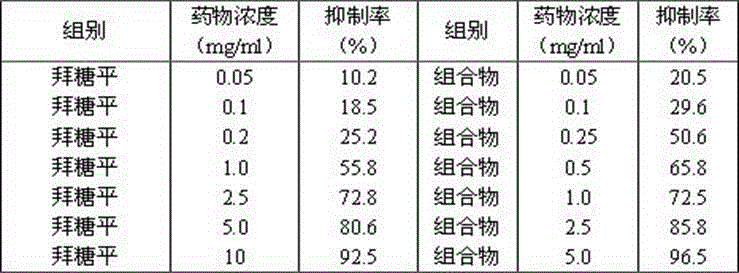
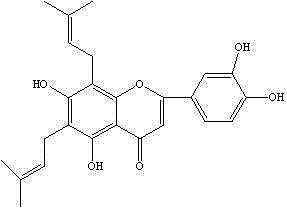
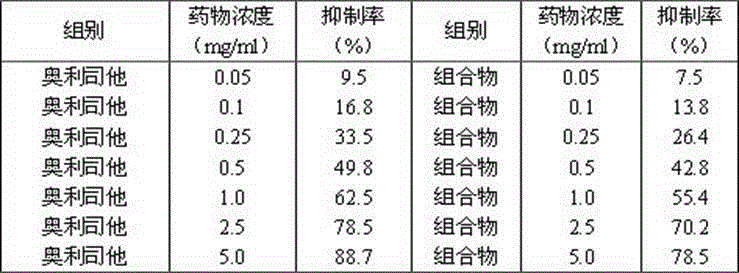
[0069] The Humulus japonicus was cut into sections, extracted twice with 70% ethanol of 8 times the weight of the medicinal material, and filtered for 2 hours each time. The filtrate passed through the treated AB-8 macroporous resin column and washed with 70% ethanol. Remove 4 times the column volume, collect the effluent and eluent, recover ethanol, concentrate, and pass the concentrated solution through an AB-8 macroporous adsorption resin column, first elute with water for 4 times the column volume, and then use 65% ethanol for 5 times. Column volume, collect the ethanol eluent, concentrate, and dry to obtain the Humulus japonicus composition, and determine 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-6,8-bisisoamyl by high performance liquid phase The content of alkenyl-4H-1-benzopyran-4-one was 55.4%, the content of luteolin-7-O-β-D-glucoside was 12.5%, and the content of apigenin-7- O- The content of β-D...
example 2
[0070] Example 2 Preparation method of 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-6,8-bis-prenyl-4H-1-benzopyran-4-one
[0071] Take the composition and dissolve it in methanol, go to C 18 ODS column, eluted with acetonitrile-water (50:50) as mobile phase, detected by thin layer, collected the target compound, recrystallized, and prepared 2-(3,4-dihydroxyphenyl)-5,7-dihydroxyl -6,8-Diprenyl-4H-1-benzopyran-4-one.
[0072] Yellow crystalline powder (methanol), mp: 198~199℃, positive for hydrochloric acid-magnesium powder reaction and positive for Molish reaction. 1H-NMR (400MHz, DMSO) δ: 12.97 (1H, s, 5-OH), 10.43 (1H, s, 4'-OH), 6.88 (1H, s, H-3), 6.44 (1H, d, J=2.4 Hz, H-6), 6.83 (1H, d, J=2.5 Hz, H-8), 7.94 (2H, d, J=8.8 Hz, H-2', 6'), 6.94 (2H, dd, J=8.8Hz, H-3', 5'), 5.07 (1H, d, J=6.0Hz, H-1 "), 3.17 (1H, m, H-2"), 4.02 (1H, m, H-3"), 3.54 (1H, m, H-4"), 3.69 (1H , m, H-5″), 4.42 (2H, m, H-6″), 5.85 (1H, d, J=15Hz, H-α), 6.79 (1H, m, H-α) β), 1.64 (3H, d, J=6.8H...
Embodiment 3
[0073] Example 3 , (capsule)
[0074] 100 g of composition raw materials, appropriate amount of medicinal starch, mixed uniformly, granulated, dried, granulated, packed into No. 1 capsules, and made into 1000 capsules. 3 capsules each time, 2 times a day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com